- JMP User Community
- :
- Blogs
- :
- JMPer Cable
- :
- New in JMP Clinical 17
The content you are looking for has been archived. View related content below.
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
The latest release of JMP Clinical has arrived! Sam Gardner (@SamGardner), Senior Product Manager for Health and Life Sciences, explains how this version of JMP Clinical has been completely refreshed to be leaner, faster, and easier to use.
Leaner: Re-engineered product uses JMP only. Previous versions of JMP Clinical used SAS for data processing and analysis. This provides a much smaller footprint for the software, making it easy to manage and deploy.
Faster: Refreshed user interface displays report filters and settings options directly within a report. Where standardization of report default settings is needed, users can adjust those settings on a per-study basis.
Easier to use: Redesigned review reports launch immediately without intermediate dialogs, allowing reviewers to rapidly see the data summaries and analysis results.
In addition, JMP Clinical 17 comes with all the advanced predictive modeling features of JMP Pro, allowing users to dive more deeply into their data.
What problems do the changes solve for our users?
This release removes many obstacles for users. The large installation packages (typically more than 12 GB) that required both SAS and JMP often led to lengthy and complicated download and installation processes. The new JMP Clinical installation package is now only 1 GB for easier deployment. Additionally, JMP Clinical is now available for both Windows and macOS.
JMP Clinical reports are faster than ever. Creating a report in previous versions required using a complicated launch dialog and long pauses waiting for backend SAS processing before the report would be created. Now reports launch very fast, often with less than a few seconds of processing time. This speed is possible because all data processing and analysis are performed in JMP alone. The new simplified reports have predefined preferences that eliminate the guesswork when creating reports, but each report also offers dynamic options for filtering, switching, grouping, and configuring the graphics and analysis. Many of these reports can be published to JMP Live 17.
Can you give an example of how the experience might be for a JMP user?
JMP Clinical continues to leverage the CDISC data models SDTM and ADAM. Users simply point to their CDISC data using JMP Clinical, load a study, and create their own clinical trial review reports. Some examples of these reports include:
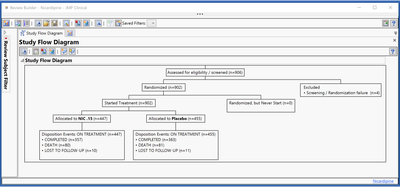
New Study Flow diagrams (or CONSORT diagrams) give a high-level visual summary of patient disposition in the trial. Clear and detailed reporting of the flow of participants through each stage of the trial is essential to assess the validity and generalizability of the trial findings, and these diagrams are expected by regulatory agencies as part of the clinical efficacy and safety section for new drug applications.
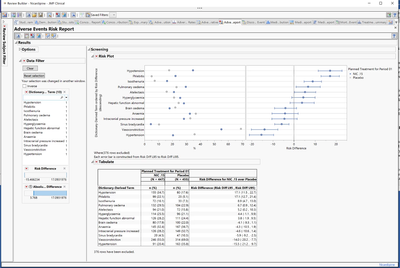
The new Adverse Events Risk Report provides a way to easily understand which adverse events are prevalent in the study treatment arms, or other study grouping categories, for easy identification of safety or efficacy signals in the data that warrant further evaluation.
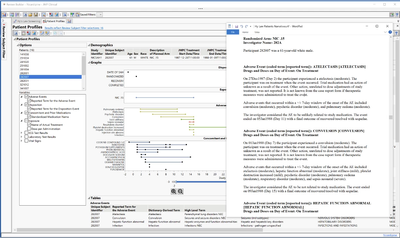
Patient Profiles in JMP Clinical 17 have been redesigned, providing both visual and graphical summaries of the subject's progress through the trial. Automated AE narratives can be generated rapidly for further documentation or standardized reporting of the subject-level results.
What are the impacts this can make on users’ work and on organizations?
JMP Clinical 17 has already had a big impact on our customers. It is used by multiple regulatory agencies in new drug application reviews. Pharmaceutical customers appreciate how JMP Clinical enables a broad range of users (not just biostatisticians) to access clinical trial data and visualize the results. It allows users to focus more on the data and less on writing code to generate visualizations and analysis because we have done the heavy lifting for them. At the same time, it is easy to create custom reports and make them available within the Clinical Review Builder, allowing more advanced programmers and data analysts to leverage JMP Clinical as the framework for all their organization’s clinical trial review needs.
What feedback have you heard from early adopters about this?
Many long-term JMP Clinical customers are excited about this new version. They believe it will simplify their deployments and allow them to make clinical reviews faster and easier.
What is next for JMP Clinical?
We continue to develop this new version of JMP Clinical, and in 2023, more clinical review reports and features will be available.
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.
- © 2024 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- About JMP
- JMP Software
- JMP User Community
- Contact