Licensing JMP® for Faculty
The Academic Suite annual license is ideal for classrooms, labs, distance learning, and other educational settings. Whether used for an individual professor’s course(s) or lab, an entire department, or across campus, the Academic Suite license provides unlimited access to faculty and students.
Your university may have a license.
Academic Suite License - Multi User
JMP® Pro
Provides access to JMP Pro for an unlimited number of academic users (including student and faculty home use) at a degree-granting institution. For use in individual or multi-user classrooms, labs, and research groups.
Includes JMP Pro for Windows, Mac, and JMP for the Cloud*; JMP Technical Support; free version upgrades; complimentary virtual and in-person** training workshops; and access to available JMP On-Demand courses through the JMP User Community.
*JMP for the Cloud requires a Windows server OS. **In-person workshops must be requested 4-6 weeks in advance and are subject to availability.Student Subscription License
JMP® single-user 12-month student license
This option is designed for courses at degree-granting institutions that don’t have access to JMP Pro, which is included with the Academic Suite license. JMP Student Subscription is a standard version intended for student and instructor use in an introductory or intermediate level course. It is available at jmp.com/student.
Visit the JMP Student Subscriptions page for details, or see a listing of textbooks.
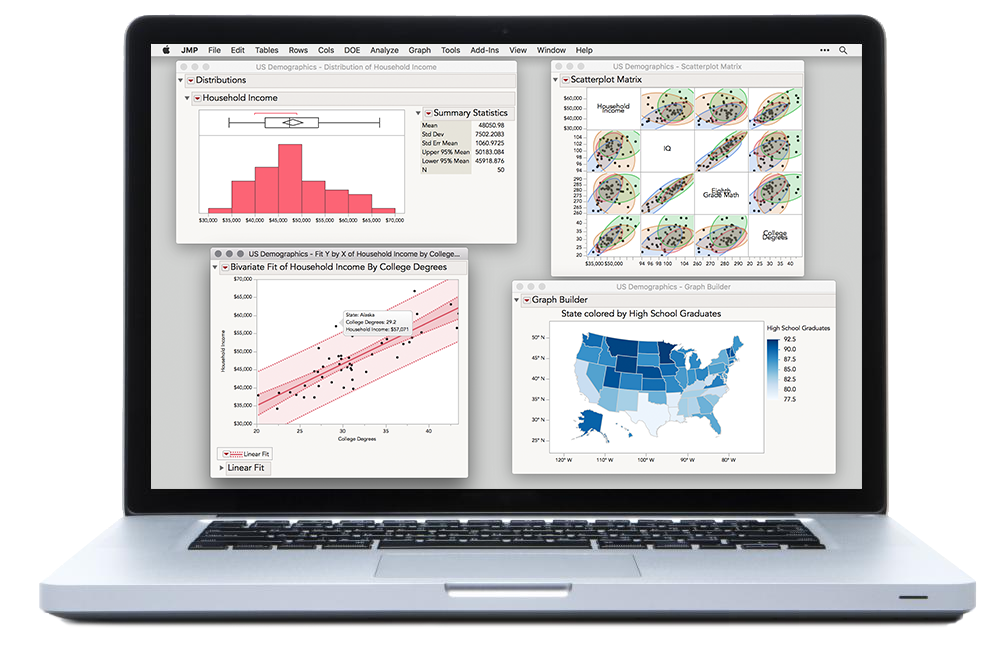
JMP®
JMP is ideal for coursework in a broad range of subjects, including introductory statistics, regression, linear models, design of experiments, quality control, reliability, multivariate analysis, predictive modeling, and applied forecasting.
JMP® Live
JMP Live is collaborative analytics software that offers a robust online platform to share interactive reports with anyone in an organization.
JMP® Pro
JMP Pro includes all of the features of JMP, plus advanced modeling techniques used in research methods, machine learning, and predictive modeling courses.
JMP® Student Subscription
JMP Student Subscription (formerly known as JMP Student Edition) is designed to meet the needs of introductory and most intermediate statistics courses offered at secondary and post-secondary academic institutions.