See JMP Clinical in action
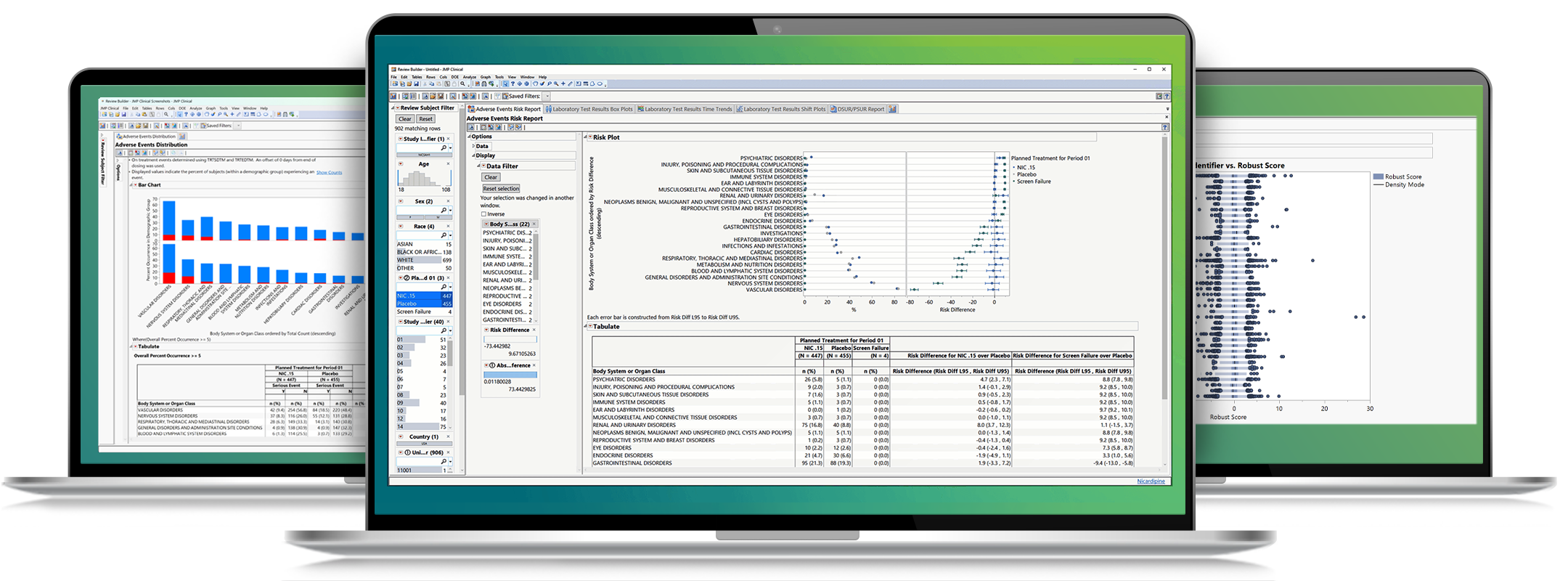
Interactively explore and understand clinical trial data
Clinical data scientists and medical monitors
Summary dashboards in JMP Clinical enable medical reviewers to evaluate safety and efficacy issues with the click of a button. Create interactive reports of adverse events, concomitant medications, labs, and vital signs; drill down to customized patient profiles and patient narratives.
Medical writers
Producing adverse event narratives for clinical study reports (CSRs) can be a painstaking, time-consuming process with significant consequences for inaccuracies or delays. With JMP Clinical, medical writers can automate patient profiles and patient narratives to reduce the time and complexity of creating output for review and submission to regulatory agencies.
Clinical operations
The goal of clinical operations is to mitigate data quality risks that could hinder a regulatory submission or drug approval. Risk-based monitoring tools in JMP Clinical help you identify data anomalies at the vendor, monitor, site, and country level to determine the factors responsible for lapses in safety or data quality.
JMP Clinical has the potential to, frankly, do anything needed for conducting and reporting clinical trials, whether it is central statistical monitoring, signal detection, narrative creation, building clinical data warehouses or, indeed, full-blown clinical trial reporting with close to a single push of a button.
Ferring Pharmaceuticals
Egbert A. Van Der Meulen, Senior Director of Biostatistics
The Core Capabilities of JMP Clinical
Patient Narratives
Automatically compose a configurable patient narrative for each subject who experienced an adverse event (AE), including reporting for deaths, serious AEs, AEs of special interest, and AEs resulting in study discontinuation.
Patient Profiles
Instantly generate customizable patient profiles, and easily communicate findings among review groups.
Data Visualization and Analysis
Reveal trends and outliers, and accelerate discovery via interactive visualization and sophisticated pattern detection.
Data Monitoring
Assess trial data for safety issues with the click of a button, and create interactive reports of adverse events.
Data Integrity
Discover data anomalies at the site and patient level, whether due to fraud, data quality, or protocol errors.
Data Management
Visually identify modifications to data, and isolate data entry and EDC errors for downstream users of the data.
Risk-based Monitoring
Reduce costly on-site source data verification while preserving data integrity and the safety of study participants.
Tumor Response Analysis
Quickly identify efficacy signals in solid tumor clinical trials using visualizations tailored to RECIST criteria, including survival plots, swimmer plots, waterfall plots, and spider plots.
DSUR/PSUR
Save time and effort in assembling the components for these required reports through the automated generation of relevant tables and listings in .rtf and/or .pdf formats.
Medical Query Reports
Identify diseases or complexes that may arise due to ongoing treatments using standardized or customized medical queries.
Interventions
Track patient exposure to treatment, identify drug interactions, and analyze distributions, event rates, and risk.
Events
Determine the onset of an adverse event and its outcomes, and discern event patterns across treatment groups.
Findings
Quickly evaluate efficacy and safety, and identify potentially harmful symptoms, including liver toxicity.
Biometrics and Biostatistics
Dig deep into clinical trial events, findings, and interventions using sophisticated statistical algorithms.