Combine Studies
This report creates a new study by combining two or more existing studies.
Running this report preserves all original data from studies to be combined, and creates new input data sets by appending rows from each corresponding parent data set. The USUBJID values of the new input data sets are unchanged. The new input data sets are then used to add the combined study to JMP Clinical.
To combine two studies:
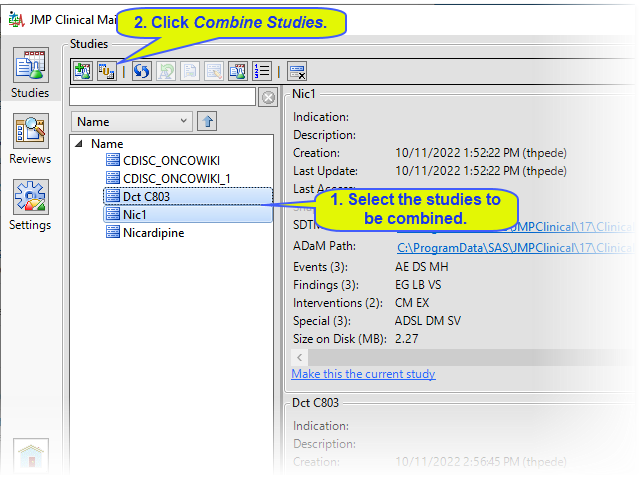
| 8 | Select the two studies to be combined and click  . . |
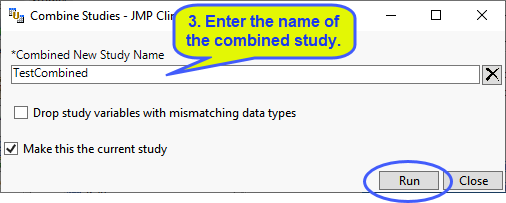
| 8 | Specify the names of the new study and the SDTM and ADaM folders and click . |
Links to specific documentation for each of the options are provided in the following table:
|
Options |
Information |
|||
|
||||
|
||||
|
Make this the current study |
|
| 8 | Specify the desired options and click . |
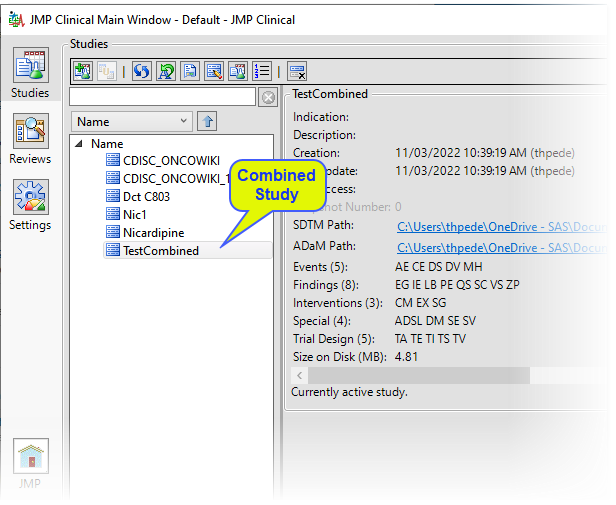
When the combination process is complete, the following Results window appears.
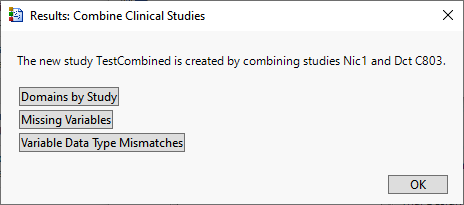
You can choose among the options to view selected items in the combined study.
The combined study has been added to the list of available studies.