Adverse Events Narrative
This report is used to generate adverse events narratives for clinical study reports.
Report Results Description
Running this report with the Nicardipine sample setting generates an rtf file containing narratives like the one below.
| 8 | Click to create an RTF of the narratives. |
The narrtext dataset output by the AE Narrative report contains variables that are used by the template to create the narrative. Refer to The Adverse Events Narrative NarrText Derivations Table for additional details about these variables and their derivations.
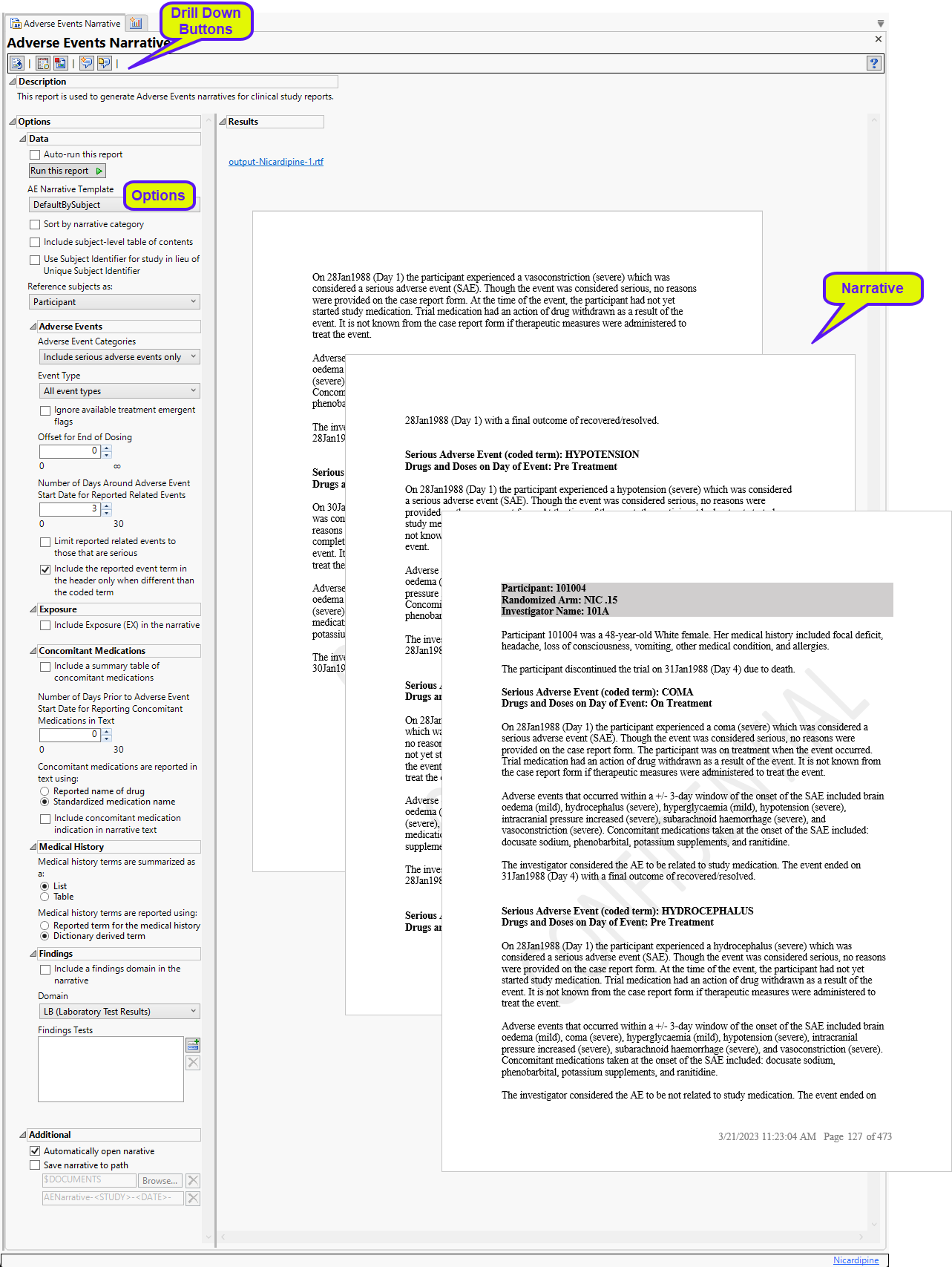
A narrative, containing descriptions of all of the observed adverse events, is generated for each patient. The narrative for the Nicardipine example is 473 pages long. In the example shown here, subject 101004 experienced 5 adverse events that are described over 3 pages.
Each narrative contains a summary of patient demographics and the description of the adverse events experienced by that patient. Each description consists of the name and occurrence of the event. followed by three paragraphs of text.
| • | Paragraph 1 describes the particular AE of interest, such as the date of occurrence, whether it met the definition of serious or not and the particular criteria (all non-serious AEs are considered Other Significant Adverse Events) and the action taken with study medication as the result of the event. Other details include the dose of drug taken at the time of the event and how long the subject was at this dose. If the event occurred between the first and last day of dosing (inclusive), and a subject received a dose of 0 or no drug taken at the time, then this is considered a dose interruption. Events before the first day of dosing are considered "Pre-treatment", while those after the last day of dosing are considered "Post-treatment". |
| • | Paragraph 2 reports on other adverse events that occur close to the start of the event of interest. The severity or toxgrade of these events are reported. Windowing is set to 3 days. Only unique instances based on AE term and severity are printed. For example, if a subject has two mild headaches in a two-day period, this is printed only once. Any medications that are taken at the time of the event are reported. |
| • | Paragraph 3 describes the causal effect of the study medication to the AE and whether the event was resolved. |
Note: Microsoft Word is the only supported word processor for viewing Adverse Events narratives. You might need to set Microsoft Word as your default word processor to prevent other word processors from opening the narratives.
Note: For information about how treatment emergent adverse events (TEAEs) are defined in JMP Clinical, please refer to How does JMP Clinical determine whether an Event Is a Treatment Emergent Adverse Event?.
Note: The following notes: Multiple treatment periods have been detected and displayed. and Pre-treatment has been assigned to period=0. are inserted at the top of the report when these events are detected in your data.
In case of incomplete or missing dates:
Asterisks after text in Narratives have the following meaning:
| • | When an incomplete/missing date is identified (xxDTC for LB, EG or VS), an asterisk (*) is appended to the end of the finding name or test code. You should review the findings for the appropriately reported set of observations. |
| • | When the AE start date (AESTDTC) is incomplete/missing, an asterisk is appended to the AE term. You should review all reported dates, study days, and contents for correctness. |
| • | When the AE start date (AESTDTC) is incomplete/missing, an asterisk is appended to the date in the narrative. You should review all contents of the narrative. |
| • | When the AE end date (AEENDTC) is incomplete/missing, an asterisk is appended to the date in the narrative. You should review the final outcome and narrative header information for correctness. |
| • | When any of the dosing records have incomplete/missingdates for exstdtc or exendtc, an asterisk is placed in the drug header that explains dose at time of the event, or the pre- or post-dose status. All text related to the drug should be reviewed. |
| • | When the date of completion or discontinuation (DSSTDTC) is incomplete/missing, an asterisk is appended to the date in the narrative. You should review these dates for correctness. |
| • | When either or both of the start or stop dates (CMSTDTC or CMENDTC) for Concomitant medications are incomplete/missing, an asterisk is appended to the end of CMTERM or CMDECOD (based on the selected analysis option). You should review the data for this medication for correctness. |
Options
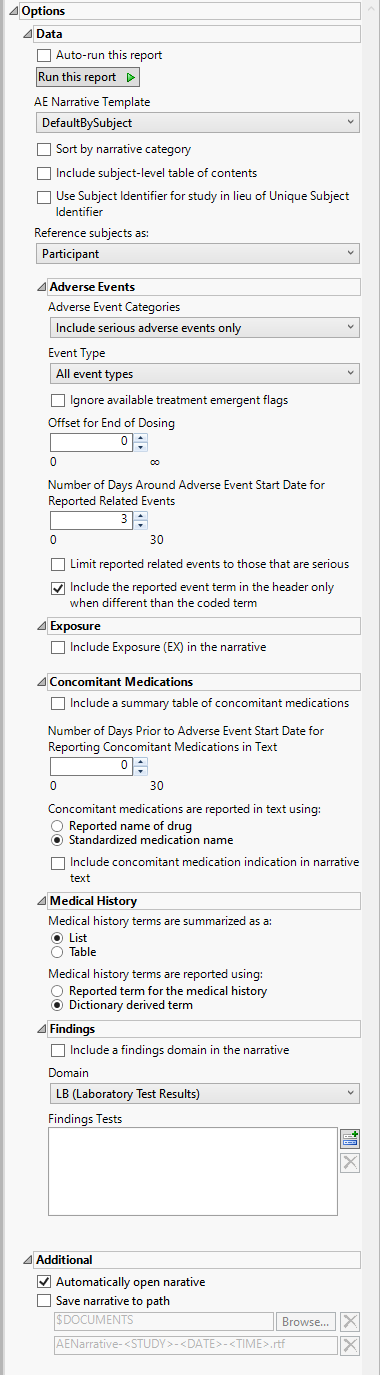
Auto-run this report
Check this box to auto-run the report upon launch. Previously saved settings are used.
Run this report
Use this widget to run this report after modifying any of the options. This option is not available when the Auto-run check box is checked.
AE Narrative Template
Use the AE Narrative Template option to select the template for the narrative. Templates enable you to customize the narrative in a variety of layouts and/or styles. The templates are read by JMP Clinical at run time and available for selection when creating the final document. The result of this design enables very customizable output that can be altered and/or extended as appropriate for different types of studies or specific regulatory requests.
Sort by narrative category
Check the Sort by narrative category option to sort the narrative by category of fatal adverse events, serious adverse events (default), adverse events leading to discontinuation, or adverse events of special interest.
Include subject level table of contents
Check this box to Include subject-level table of contents in the narrative.
Use Subject Identifier for study in lieu of Unique Subject Identifier
The Use Subject Identifier for study in lieu of Unique Subject Identifier widget enables you to use the subject identifier for study (SUBJID), if it is available, in lieu of unique subject identifier (USUBJID) in the narrative text. If SUBJID is not available, USUBJID is used as normal.
Reference subjects as:
Use this widget to specify the term used in the narratives to refer to subjects. Options include participant, subject, and patient. You may also opt to specify your term.
Adverse Events Categories
Use the drop-down menu to specify the category of adverse events to include in the narrative. Options include fatal serious adverse events only, serious adverse events only, non-serious adverse events leading to discontinuation, or all adverse events.
Ignore available treatment emergent flags
If you choose to Ignore available treatment emergent flags, the analysis includes all adverse events that occur on or after day 1 of the study.
Offset for End of Dosing
By default, post-treatment monitoring begins after the patient receives the last treatment. However, you might want to specify an Offset for End of Dosing, increasing the time between the end of dosing and post-treatment monitoring for treatments having an extended half-life.
Number of Days Around Adverse Event Start Date for Reported Related Events
You might want to include in the narrative additional events that occur at or about the same time as the reported adverse events. Use the Number of days around Adverse Event start date for reported related events option to specify the number of days before and after the onset of the adverse event, within which the additional events are considered. only those events falling within the specified time are included in the narrative.
Limit reported related events to those that are serious
By default, reporting additional adverse events that occur close in time to each summarized event includes all adverse events experienced by the patient. Use the Limit reported related events to those that are serious option limit those events to serious adverse events only.
Include the reported event term in the header only when different than the coded term
Both the coded term for the AE (AEDECOD) and the reported term are included in the headers within the narrative. You can use the Include the reported event term in the header only when different than the coded term to include the reported event term (AETERM) in the header only when different from the coded term (AEDECOD). If they are the same, only the coded term is used.
Include Exposure (EX) in the narrative
You can opt to Include Exposure (EX) in the narrative. The report sums up values of EXDOSE for each day across all records. It does not take into account units such as mg/hr or dosing frequency such as BD or QD. Instead, this report takes the simplest possible approach and assumes that the study sponsors would ultimately report the total dose for a particular study day in EXDOSE. This is also partially why including dosing into the narrative is optional.
All study treatments are to be taken at least once per day
If the study protocol that all study treatments are to be taken at least once each day you should check the All study treatments are to be taken at least once per day option. This is used to calculate drug (including dose interruptions) dosage at the time of the event. Note: This option is checked by default. You should uncheck this box if at least one drug is taken less frequently than once per day.
A 0 dose for placebo or vehicle indicates a dose interruption
The A 0 dose for placebo or vehicle indicates a dose interruption option is used when a dose of 0 for either the placebo or the treatment indicates a dose interruption. This option is specified by default.
Include a summary table of concomitant medications
Use this widget to report information about the concomitant medications study subjects are taking and their possible relationship to observed adverse effects. Note: By default, concomitant medications are not summarized in the narrative. Use the Include a summary table of concomitant medications to include this information.
Use original study days instead of derive values
When you opt to include a summary table of concomitant medications, you can also opt to Use original study days instead of derived values
Number of Days Prior to Adverse Event Start Date for Reporting Concomitant Medications in Text
The Number of days prior to Adverse Event start date for reporting concomitant medications option enables you to specify the time period on or before treatment that you want to consider. A value of 0 indicates medications that were taken the day of event onset; this value is specified by default. A value of X includes concomitant medications that were taken on the day of the onset of the AE and up to X days prior to AE onset.
Concomitant Medications are reported in text using:
Use the Concomitant medications are reported in text using: option to specify how to report the name of the drug.
Include concomitant medication indication in narrative text
Check this box to include a table concomitant medication indications in the narrative.
Medical history terms are summarized as a:
You can specify whether the Medical history terms are summarized as a table or a list.
Medical history terms are reported using:
Use the Medical history terms are reported using: widget to specify the appropriate medical history terms to be used in narrative text.
Include a findings domain in the narrative
You can opt to Include a findings domain in the narrative.
Domain
Use the Findings Domain to Analyze option to specify whether to plot the distribution of measurements from either the Electrocardiogram (EG), Laboratory (LB), or Vital Signs (VS) findings domains.
Findings Tests
Use this widget to select specific Findings tests.
| 8 | Click  to open the Add window (shown below) that lists available test names (shown below). to open the Add window (shown below) that lists available test names (shown below). |
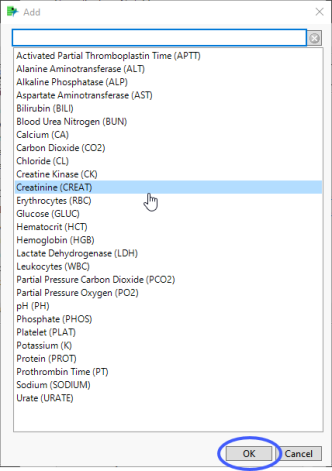
| 8 | Select the desired test(s) and click to add them to the text box. |
Include a summary of baseline findings
You might opt to Include summary of baseline findings in the narrative. Note: If the Laboratory Test Results (LB) domain has been specified, only abnormal baseline lab tests are summarized in the body of the narrative.
Include closest on-study findings test dates
Use the Include closest on-study findings test dates: option to specify whether to report results from lab tests taken prior to or on the day of the onset of the adverse event only, results of tests taken subsequent to the onset of the adverse event only, results from tests taken both before and after the onset. You can also choose Neither to include none of the lab results.
Include on-study lab tests where reference range indicator is:
Use the Include on-study lab tests where reference range indicator is: option to specify which findings results to include in the narrative.
Refer to findings tests using
Use the Refer to findings tests using: option to specify the terminology used to refer to the findings results in the narrative.
Automatically open narrative
Check this box to automatically open the rtf output file upon completion of the narrative run.
Save narrative to path
Check this box to automatically save the narrative output to the specified location. Your Documents folder is specified by default. The narrative rtf file is identified as AENarrative-<Study>-<Date>-<Time>, where <STUDY> is the name of the study and <Date>-<Time> is the date and time the narrative was run.
General and Drill Down Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Click  to rerun the report using default settings. to rerun the report using default settings. |
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
Default Settings
Refer to Set Study Preferences for default Subject Level settings.