Duplicate Records
This report identifies sets of records that have identical values on more than one occasion within a subject or between subjects within a study site. This report identifies records based on SITEID and the following covariates (if available): visit number (VISIT/VISITNUM), category (xxCAT), subcategory (xxSCAT), location (xxLOC), method (xxMETHOD), position (xxPOS), specimen (xxSPEC), and planned time point (xxTPT).
Report Results Description
Running Duplicate Records for Nicardipine using default settings generates the Report shown below.
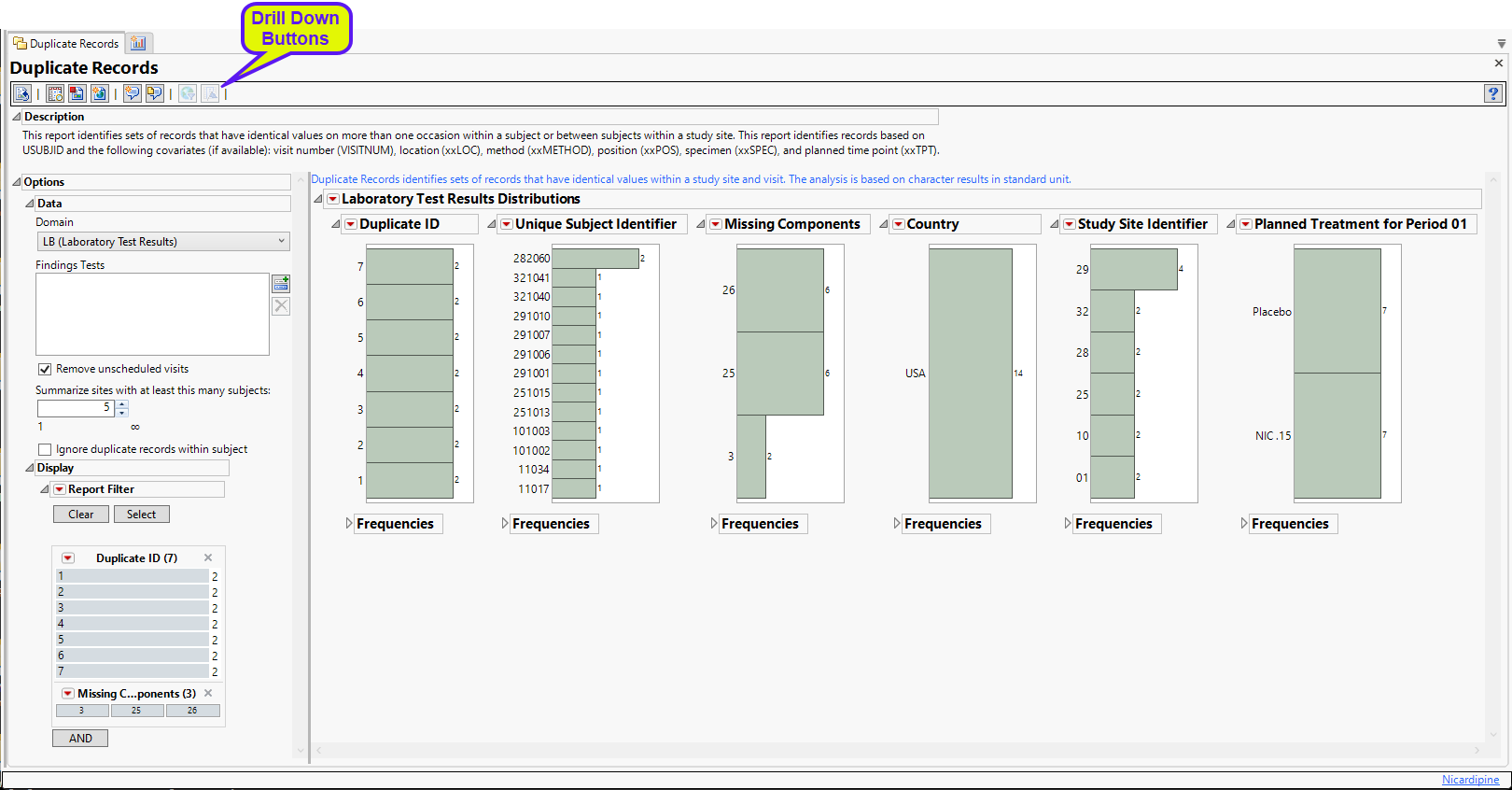
The Report contains distribution plots for the findings domain specified in Options. Data that are summarized are those test codes that are constant throughout the course of the study.
Analysis of the Vital Signs domain is shown below:
Vital Signs
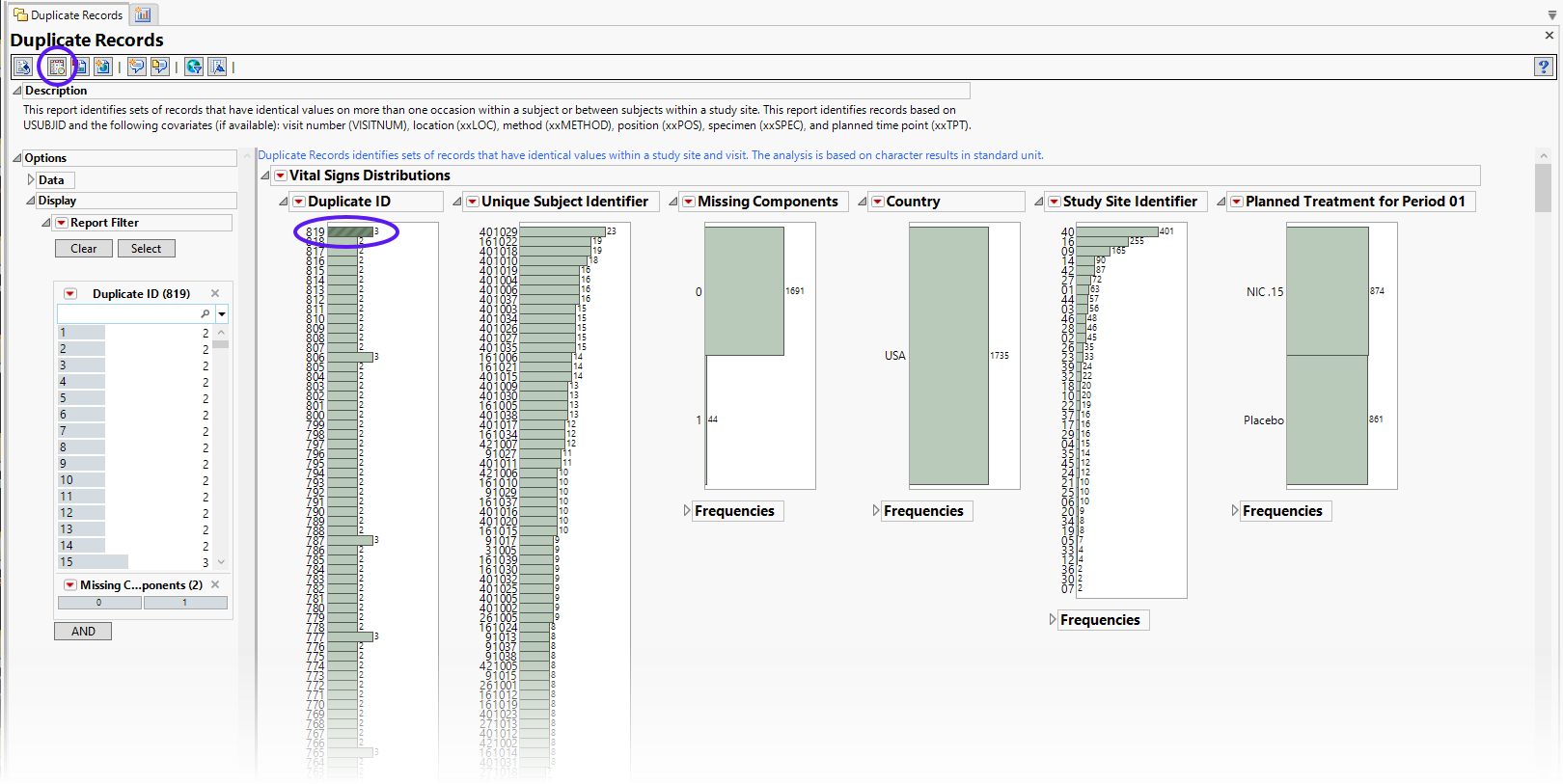
The Vital Signs section contains the following elements:
| • | One or more Histograms. |
The data table underlying these histograms represents records that have at least one duplicate within or between subjects in the trial (depending on options). Therefore, Unique Subject Identifier shows the number of subject records that occur in duplicate bins. Missing Components helps identify duplicate records with a majority of nonmissing data. For example if ALT, AST, and ALP are analyzed, and for a subject at a certain visit there is no ALT result, then the Missing Component number is 1. Country, Study Site Identifier, and Planned Treatment are provided to show where these duplicates occur most frequently.
See Distribution for more information. Duplicate ID is created only if the number of distinct Duplicate IDs is less than or equal to 3000.
Using the button shows the records for selected subjects. This details the records that are duplicated within the subject or across other subjects.
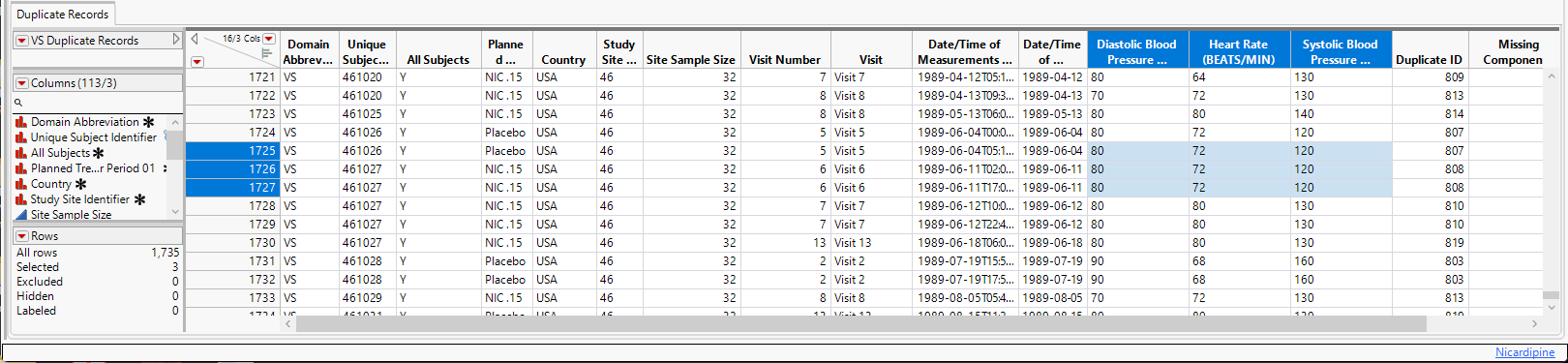
To see duplicates, select one group (shown above) and click  to view the duplicates in the associated data table The duplicates are highlighted). In these cases, each subject’s Diastolic Blood Pressure, BlSystolic Blood Pressure, and Heart Rate (highlighted columns) values are identical. It is unlikely to expect that these sets of values would be the same across subjects (or perhaps even repeat identically within subjects).
to view the duplicates in the associated data table The duplicates are highlighted). In these cases, each subject’s Diastolic Blood Pressure, BlSystolic Blood Pressure, and Heart Rate (highlighted columns) values are identical. It is unlikely to expect that these sets of values would be the same across subjects (or perhaps even repeat identically within subjects).
Options
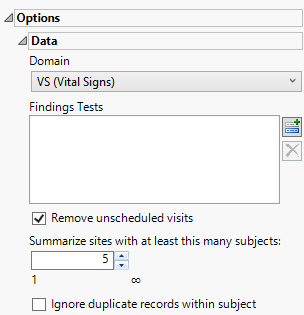
Domain
Use this widget to specify whether to plot the distribution of measurements from either the Electrocardiogram (EG), Laboratory (LB), or Vital Signs (VS) findings domains.
Findings Tests
Use this widget to select Findings Tests for the analysis. The report will autorun and analysis is restricted to the selected tests only.
Remove unscheduled visits
You might or might not want to include unscheduled visits when you are analyzing findings by visit. Check the Remove unscheduled visits to exclude unscheduled visits.
Summarize sites with at least this many subjects:
The Summarize sites with at least this many subjects: widget enables you to set a minimal threshold for the sites to be analyzed. Only those sites which exceed the specified number of subjects are included. This feature is useful because it enables you to exclude smaller sites, where small differences due to random events are more likely to appear more significant than they truly are. In larger sites, observed differences from expected attendance due to random events are more likely to be significant because any deviations due to random events are less likely to be observed.
Ignore duplicate records withing subjects
Under certain circumstances, you may want to ignore select duplicate records. For example, if duplicate entries from a visit are made for a subject, you only want to consider one set of entries. Check the Ignore duplicate records within subject box to delete multiple occurrences of the same subject within each set of duplicate records. If a set of duplicates is based entirely on one subject, these sets are removed.
Report Filter
These filters enable you to subset and view subjects based on demographic characteristics and other criteria. Refer to Data Filter for more information.

General and Drill Down Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Click  to rerun the report using default settings. to rerun the report using default settings. |
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Review Subject Filter. to open and view the Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
Default Settings
Refer to Set Study Preferences for default Subject Level settings.
Methodology
No statistical tests are performed. This report identifies sets of tests with similar values using Unique Subject Identifier; Visit number, BY-values based on xxCAT, xxSCAT, xxLOC, xxMETHOD, xxPOS, xxSPEC, and xxTPT (if selected); and date-time of collection (xxDTC) to determine sets of records.