The JMP Clinical Main Window Changes Depending on the Assigned User Role.
The appearance of the JMP Clinical Main Window on your machine depends on the specified Role Assignments to which your installation of JMP Clinical has been configured. Because of this, you might not see all of the options discussed previously and your main window might appear quite different.
What follows here is a brief description of each of the JMP Clinical user roles, and their associated dashboards, that can be assigned by your system administrator1. Please note that because user roles can be combined (a review author can also be a settings editor, for example) the main window that you see might be somewhat different than shown here.
Study Manager
A user with this role can do all of the operations involved in managing studies within JMP Clinical: add, combine, and refresh metadata, rename or move folders, update a review with a new snapshot, update the study risk data set, and delete studies.
The “Study Manager” main window is shown below:
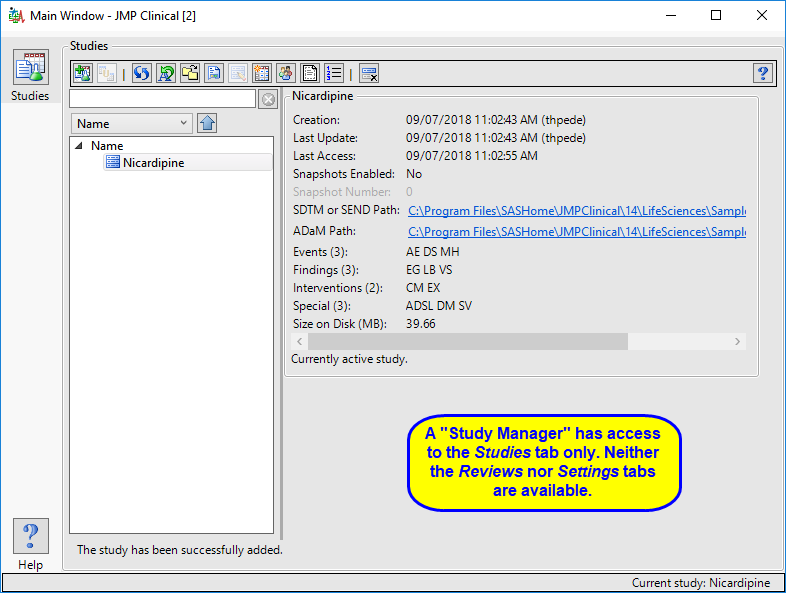
The study manager has full access to the Studies tab. Neither the Reviews tab nor the Settings tab, with their associated functions, are available to this user.
Study List Manager
A user with this role can select a study, view its snapshot history, explore the subjects in the study. They can also delete a study when the data files associated with it have been deleted.
The “Study List Manager” main window is shown below:
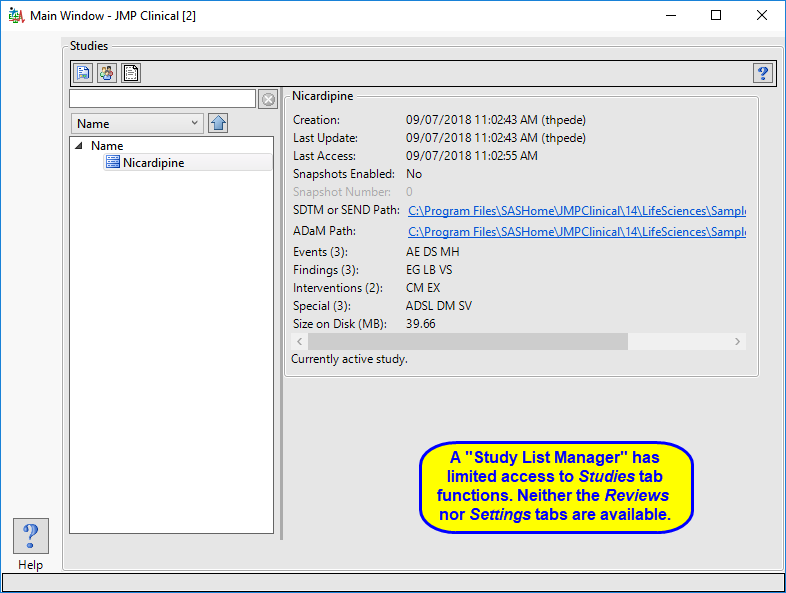
The study manager has limited access to the Studies tab. Neither the Reviews tab nor the Settings tab, with their associated functions, are available to this user.
Review Author
A user with this role can create/edit/save Review Templates, do ad hoc analyses, and create and save Reviews. They can also manage the Holiday and JMP Life Sciences / SAS Institute Event data set and Risk Threshold data sets used by the configuration. A Review Author implicitly has the Reviewer role as well.
The “Review Author” main window is shown below:
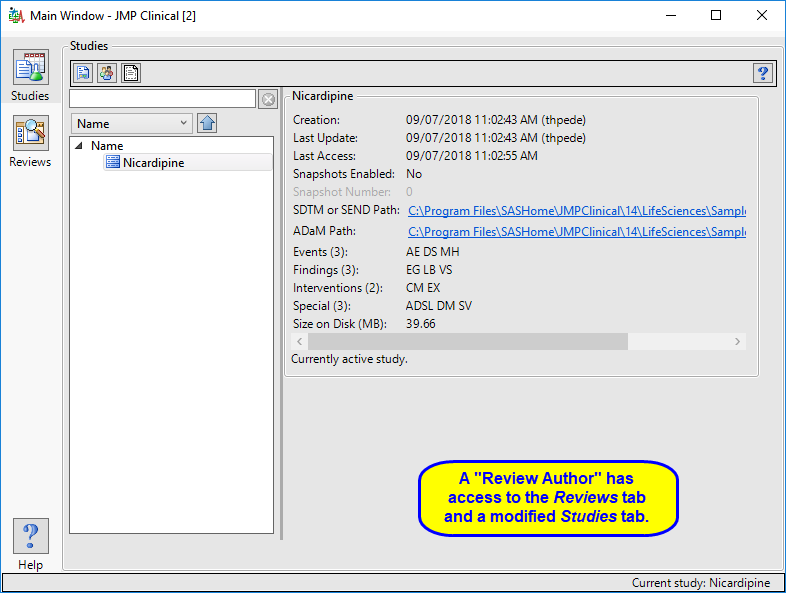
The review author has access to a modified Studies window where they can select a study, view its snapshot history, and explore the subjects in the study. Review authors can access all functions on the Reviews tabs and author new reviews and open existing reviews. The Settings tab is not available.
Reviewer
A user with this role can open and interact with the finalized Reviews that have been created for them. Typically, the Reviewer does not have Author or Study Manager rights and thus cannot make changes to a review. Someone with Reviewer (and Settings Editor) have a very simple interface (as shown below), with NO Studies or Settings tabs and no way to access study information or the file path directories.
The “Reviewer” main window is shown below:
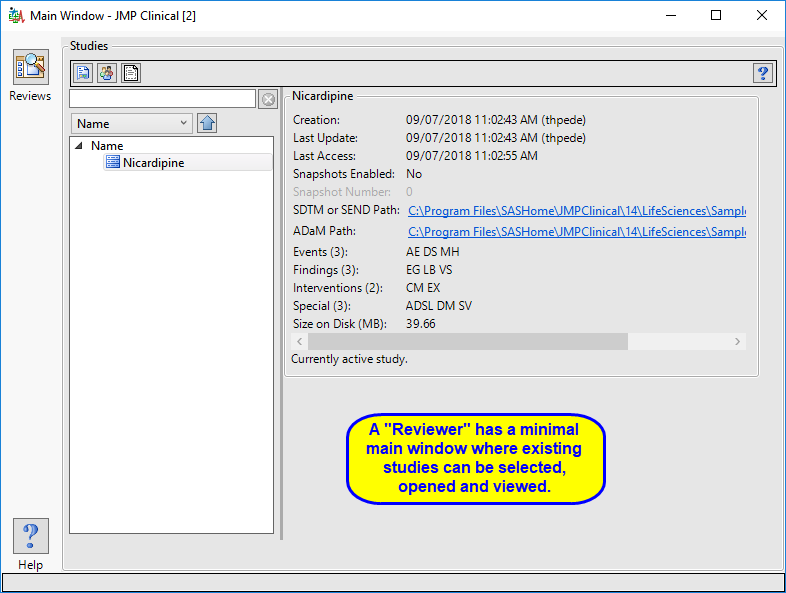
The reviewer has access to a modified Studies tab where they can select, open, and view existing reviews.
Configuration Manager
A user with this role can add and define JMP Clinical configurations. This role is assigned to all users by default.
Settings Editor
This role is normally found in conjunction with one or more of the other user roles. A user with this role has access to the Settings tab on the Main Clinical window. This enables them to select different configurations (if defined), change how they view Documentation and Help, and download and install a local copy of the documentation.
The “Settings Editor” main window is shown below:
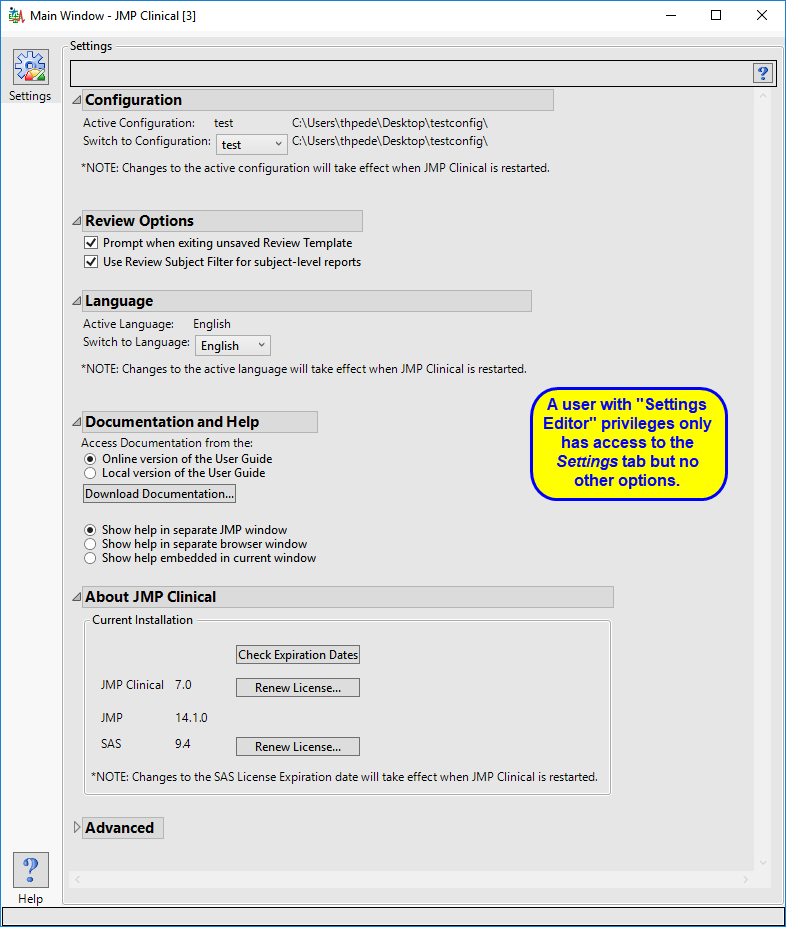
The settings editor has access to the Settings tab only.
Combined User Roles
JMP Clinical can be configured such that individual users have multiple roles. The Main Window surfaced to those users are permissive and show all the options available for each role.