Term Level
Use this option to specify which level of terms (for example, adverse event, intervention, or MedDRA) to use as categories.
Possible term levels are report-specific and are listed in the following table:
|
Term Level |
Corresponding Variable |
Function |
||||||
|
Preferred Term (PT) |
AEDECOD in AE |
|
||||||
|
Verbatim Term (VT) |
AETERM in AE |
|
||||||
|
Body System |
AEBODSYS in AE |
|
||||||
|
High(er) Level Term (HLT) |
AEHLT in AE |
|
||||||
|
High(er) Level Group Term (HLGT) |
AEHLGT in AE |
|
||||||
|
Standard MedDRA Query |
|
|
||||||
|
Primary System Organ Class (SOC) |
AESOC in AE |
|
||||||
|
Lowest Level Term (LLT) |
AELLT in AE |
|
||||||
|
Body System or Organ Class |
AEBODSYS in AE |
|
||||||
|
Dictionary-Derived Term |
AEDECOD in AE |
|
||||||
|
Reported Term for the Adverse Event |
AETERM in AE |
|
||||||
|
Reported Name |
either CMTRT (for Concomitant Medications) or SUTRT (for Substance Use) |
|
||||||
|
Standardized Name |
either CMDECOD (for Concomitant Medications) or SUDECOD (for Substance Use) |
|
Note: Input data determines the availability of options. Not all term levels are available in all of the reports in which term levels are specified.
Note: The dictionary name and version used to generate the descriptions should be included as part of the submission.
For Interventions
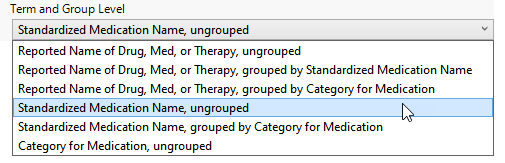
Possible term levels are report-specific and are listed in the following table:
|
Term Level |
Corresponding Variable |
Function |
|||
|
Reported Term for the Drug, Med, or Therapy |
CMTRT in CM |
|
|||
|
Standardized Medication Name |
CMDECOD in CM |
|
|||
| Category for Medication |
CMCAT in CM |
|