This report creates tabular and graphical overviews of treatment emergent
adverse events
for the safety
population
by actual treatment
arm
.
Running
Treatment Emergent AE Summary
for
Nicardipine
using default settings generates the
Report
shown below.
The
Results
window contains the following panes:
|
•
|
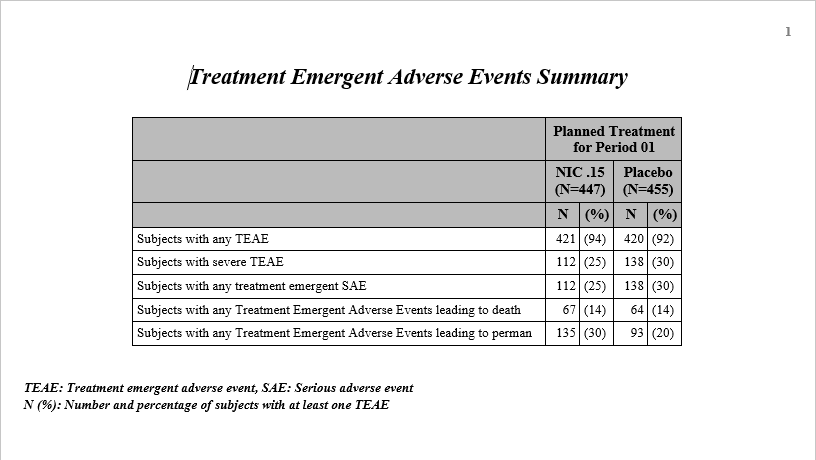
Summary Chart
: This tab contains a
Bar Chart
summarizing the counts of various treatment emergent
adverse events
by treatment. Click the
Open TEAE Summary.rtf
link to open an rtf file (shown below) that summarizes the treatment emergent adverse events.
Percentages
of subjects experiencing an event by
arm
are also included in this summary.
|
This enables you to subset subjects based on demographic characteristics and study site. Refer to
Data Filter
for more information.
|
•
|
Profile Subjects
: Select subjects and click
|
|
•
|
Show Subjects
: Select subjects and click
|
|
•
|
Cluster Subjects
: Select subjects and click
|
|
•
|
AE Narrative
: Select subjects and click
|
|
•
|
Create Subject Filter
: Select subjects and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the
arrow to reopen the completed process dialog used to generate this output.
|
Note
: For information about how treatment emergent adverse events (TEAEs) are defined in JMP Clinical, please refer to
Determining If an Event Is a Treatment Emergent Adverse Event
.