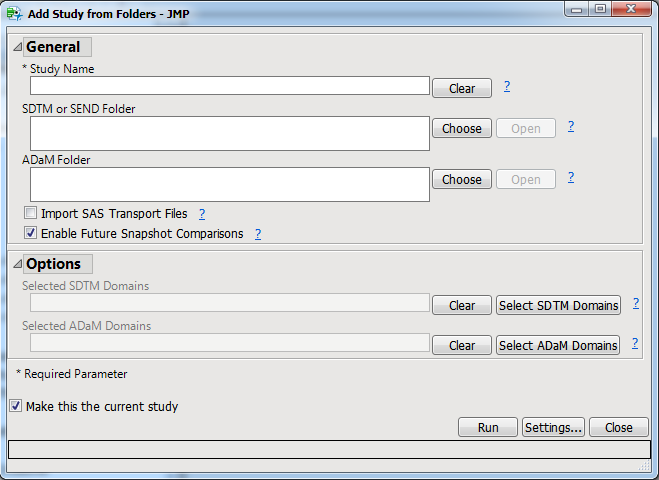
This process adds a study (see
Studies
) from folders that contain
CDISC
-formatted data. You must add a study before you can perform the analysis. This process can also import
SAS transport files
for you.
|
|||||||||||
|
Note
: If your JMP Clinical software has been configured for SAS Drug Development, clicking
 expands a drop-down menu. Select
From Folders
from the drop-down menu to open the
Add Study
dialog.
expands a drop-down menu. Select
From Folders
from the drop-down menu to open the
Add Study
dialog.
The study and associated
metadata
is added to your database.
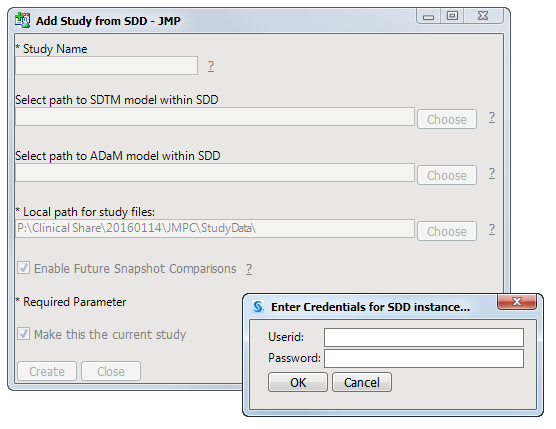
Note
: You must be a registered user of SAS Drug Development software to add a study from SDD. Your JMP Clinical software must also be configured for accessing SDD. Refer to
How do I configure JMP Clinical to access data from SAS Drug Development (SDD)?
for more information about configuring JMP Clinical for SDD.
|
|
The study and associated
metadata
is added to your database.
Refer to
How does JMP Clinical identify domains included in a study?
for information about how
JMP Clinical
assigns domains.