All study information must be recorded using an
ADSL
data set following the
ADaM
standard to support multiple treatment periods.
|
•
|
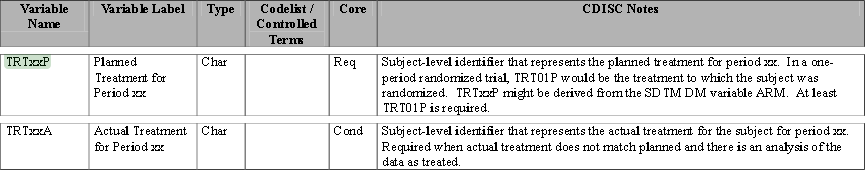
Treatment Variables
TRTxxP
or
TRTxxA
: The Planned or Actual treatment for a given treatment period
xx
(The
TRT01P
,
TRT02P
, for example, would be the variables used to record the planned treatment for a two-period crossover analysis).
|
|
•
|
Timing Variables
(
TRxxSDT
or
TRxxSDTM
) and (
TRxxEDT
or
TRxxEDTM
): Dates or Date/Times in numeric format indicating the start and end dates respectively for each treatment period
xx
.
|
Refer to the
ADaM Implementation Guide
for additional information.
The system detects a crossover if multiple
TRTxxP
or
TRTxxA
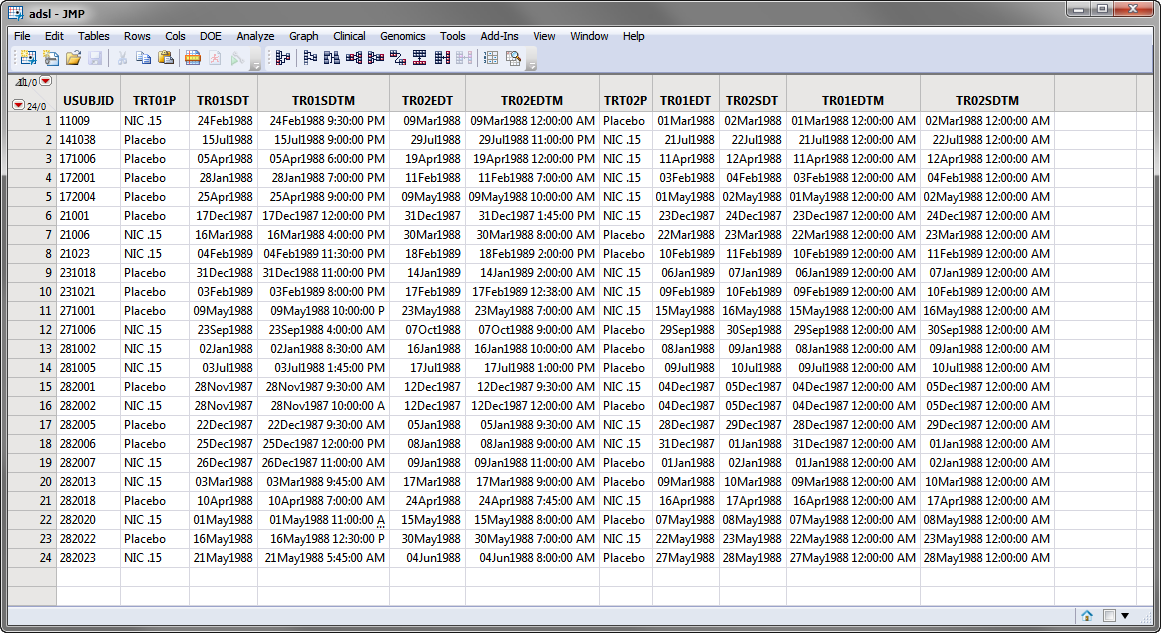
variables exist and the associated timing variables for the xx period exist and contain values. Those values might look like the values in the Nicardipine Cross-over sample data set, shown below.
Note
: For clarity, the majority of the
adsl.sas7bdat
columns in the screen shot below above have been hidden using the
Cols > Hide/Unhide
command.
In the screen shot of
adsl.sas7bdat
, shown above, the
SAS variable names
are shown. During the report analysis, when
adsl.sas7bdat
is merged into the relevant analysis domain (for example,
AE
,
LB
,
VS
), the
adsl
treatment timing variables are compared to the timing variable in the domain and new variables:
Treatment
,
Period
, and
Treatment (Period)
are created. The values for these variables are assigned based on the value of the
TRTxxP
or
TRTxxA
and the value of the
xx
in the variable name when the start date of the domain record falls within the treatment period dates.
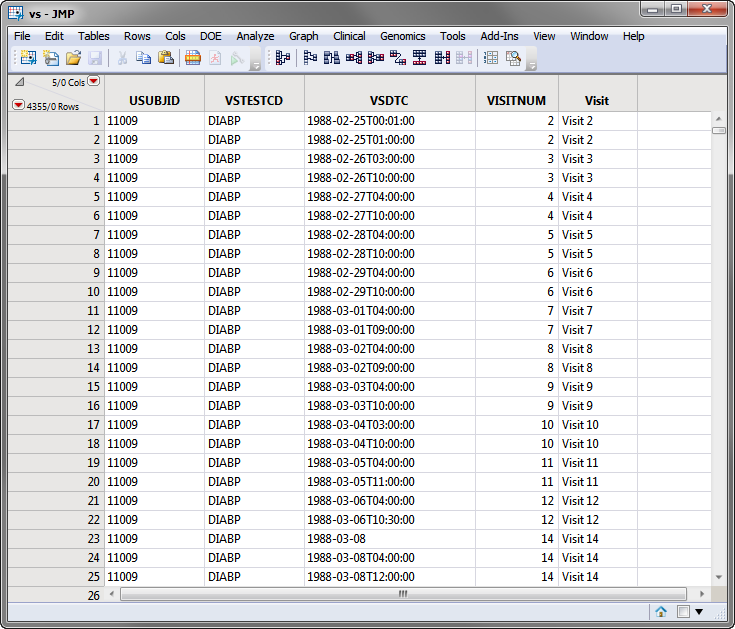
The
SDTM
data records for a subject can appear as shown in the portion of the
VS
domain (
vs.sas7bdat
(SAS names are being shown)) for the Nicardipine Cross-over sample data set, shown below.
Note
: For clarity, some of the
vs.sas7bdat
columns in the screen shot below above have been hidden using the
Cols > Hide/Unhide
command.
Based on comparison of the
VSDTC
date/time (note that SDTM follows the ISO 8601 date/time standard) with the ADSL timing date/time (numeric SAS date format), the subject records are assigned to the first treatment for Visits 1-6 and the second treatment period for Visits 7-14.
Similar analyses are done for events and intervention domains. In these domains, the timing comparisons for the record is based on the start date/time. For example, with the AE domain the AESDTC variable is used to assign the treatment period; indicating that the
adverse event
must START within the treatment period in order to be assigned that treatment value.
A sample Nicardipine Crossover analysis is now shipped with JMP Clinical. This can be loaded (using the
button) from the
Add Study from Folders
dialog
from the Clinical Starter Menu.