The
AE Distribution
report is used as an example to describe the analysis performed. This report has the most sophisticated
dialog
options of all the
distribution
reports for customizing the resulting reports. Other Events/Interventions domains follow a similar, although simpler,
workflow
.
AE Distribution
requires (or expects) several demographic- and specific domain-related
variables
to generate full report results. The system is flexible. If a certain variable does not exist, the analysis is still performed without it whenever a related variable can be substituted. For example, in
adverse event
, required, or expected, variables include:
|
•
|
USUBJID
,
|
|
•
|
a treatment variable (examples include either
TRTxxP
or
TRTxxA
,
ARM
, or a specified comparison variable
1
),
|
|
•
|
|
•
|
Treatment Date/Time variables (required if filtering interventions/events based on study treatment). These can include
TRTSDTM
,
TRTSDTC
,
TRTEDTM
,
TRTEDTC
(
ADSL
) or
RFXSTDC
,
RFSTDTC
,
RFXENDTC
,
RFENDTC
(DM domain),
EXSTDTC
, and
EXENDTC
(EX domain),
|
|
•
|
|
•
|
AESTDTC
(Required),
|
|
•
|
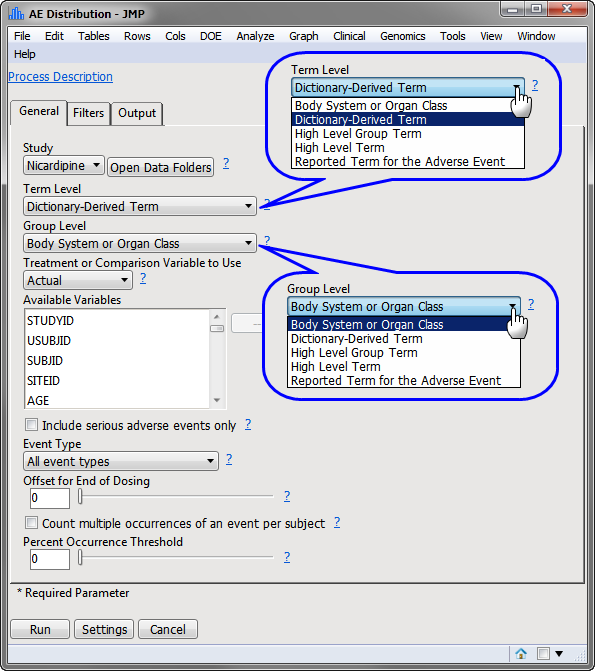
The
AE Distribution
dialog (shown below) enables you to use the term level and group level that are available in your
ae.sas7bdat
data so that while
AEDECOD
and
AEBODSYS
are specified by default variables in the examples shown here, this specification can be customized based on the term levels available in the given Study.
NONE
can also be specified here.