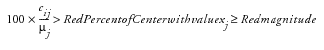|
|
Click any of the “
?
” links in the analysis
dialogs
to access the relevant Help page for a particular term or specification.
|
|
|
Click the
Process Description
link at the top of any analysis dialog to see a description of the analysis, including requirements.
|
|
|
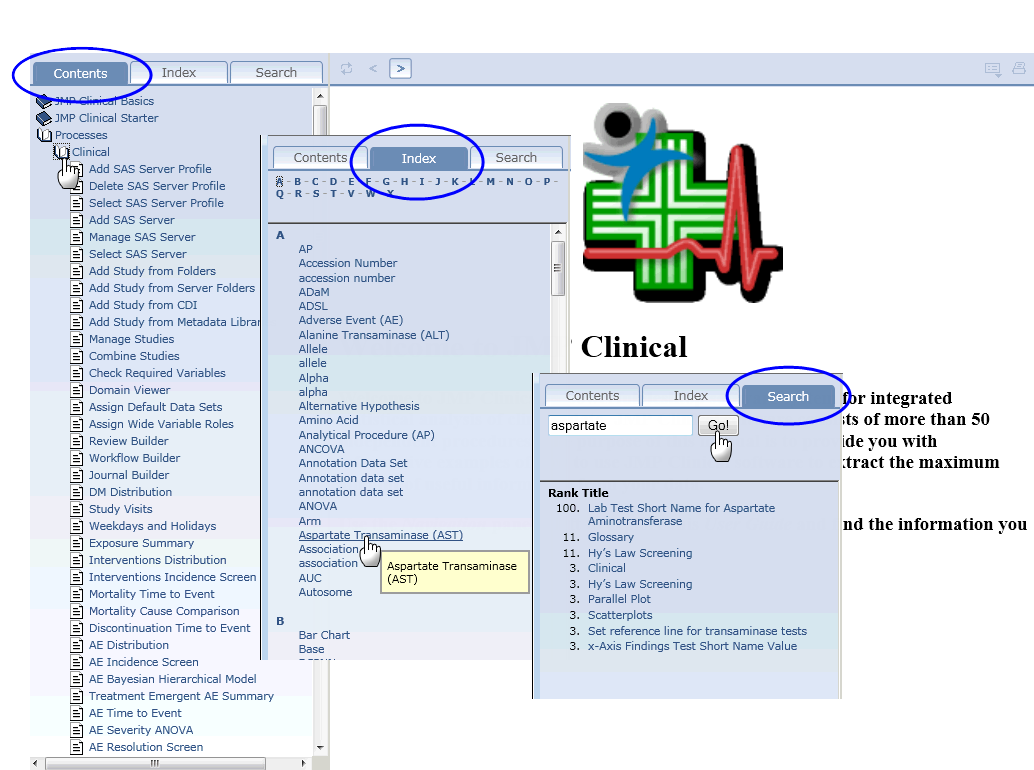
To find specific information within the Help system, use either the Table of
Contents
, the
Index
, or the
Search
options, as shown below:
Times and dates are an integral part of the data generated in all clinical trials. At least one timing
variable
must be included in all
SDTM
subject-level domain data sets. Time and date variables are
numerically formatted
according to the following ISO 8601 standard:
YYYY-MM-DDThh:mm:ss
, where
|
•
|
YYYY
is the four-digit year,
|
|
•
|
MM
is the two-digit month (values rage from 01-12),
|
|
•
|
DD
is the two-digit day (values range from 01-31),
|
|
•
|
hh
is the two-digit hour (values range from 00-23)
|
|
•
|
mm
is the two-digit minutes (values range from 00-59), and
|
|
•
|
ss
is the two-digit seconds (values range from 00-59).
|
|
•
|
T, which
indicates that time information is included (omitted if no time component is included),
|
|
•
|
-
, which either separates the date elements or can be used to indicate missing date components
|
|
•
|
:
, which separates time elements,
|
|
•
|
/
, which can be used to separate the date components from the time components, and
|
|
•
|
P
, which serves as a duration indicator and precedes the date/time components representing the duration of an event or intervention.
|
Note
: Spaces are never allowed in any ISO 8601-formatted representations of dates/times.
Dates and times can be and are expressed as complete dates/times, partial date/times, or incomplete date/times. JMP Clinical recognizes each of these elements and handles partial or incomplete dates/times as described in the separate FAQ:
How does JMP Clinical handle partial or incomplete date and time information?
Domains are evaluated for SDTM folder and ADaM folder. Domains from SDTM folder are named using the 2 letter code
XX
, where
XX
. can be any two letters. For example, the domain containing adverse event data is
AE
(the data set name).
Domains from ADAM always contain
AD
as the first two letters of the domain name. ADSL is constant, while other letters following the
AD
identify the specific domain. For example,
ADAE
is the ADaM domain
AE
.
Domains are classified as findings if
XXTESTCD
is present, interventions if
XXTRT
is present, or events if
XXDECOD
is present. If domain type cannot be identified for a given folder, the domain is ignored.
In addition, Basic Data Structure (BDS) is supported for ADAM. If
PARAMCD
and either
AVAL
or
AVALC
are present, the domain is considered a findings domain. If other variables are present as above and the domain type cannot be identified, it is ignored. This is true even if
XXTESTCD
is present since it would not be clear whether to transform ADaM variables or use SDTM variables for the domain.
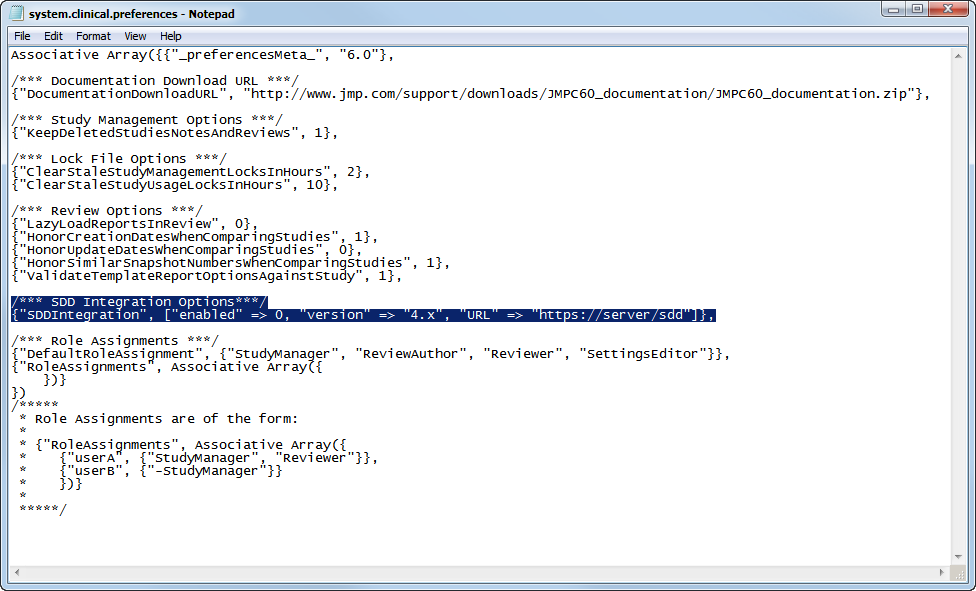
You must modify the
system.clinical.prefereneces
file that is found in all of your local and shared configurations in order to access SDD. Locate the file in the configuration that you want to use to access SDD (the default local location for this file is the
C:\Program Files\SASHome\JMPClinical\12\LifeSciences
directory) and proceed as follows:
|
|
Open the
system.clinical.prefereneces
file with a text editor.
|
|
|
|
|
To specify the url of the SDD server, replace the italicized text in the following
“URL” => “
https://server/SDD
”
with the url for your SDD server.
|
Note
: You must include the quotation marks as shown in the text above. Entries are case-sensitive.
|
|
Save and close the
system.clinical.prefereneces
file.
|
There are two files in your JMP Clinical installation folder (normally
C:\Program Files\SASHome\JMPClinical\12\LifeSciences
) that tell JMP Clinical where to look for your studies and where the output data, reports, notes, and so on are to be placed.
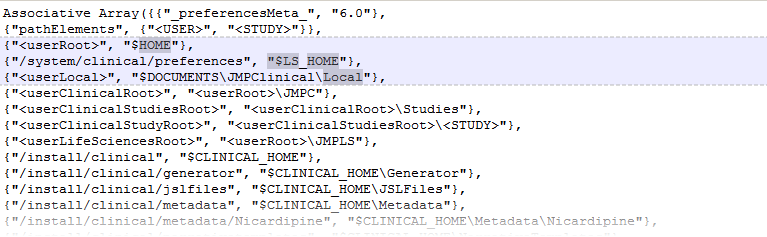
The first of these files,
installation.path.preferences
tells JMP Clinical where to find the file paths to the locations of all of your studies and other need information. A portion of the default
installation.path.preferences
file is shown below:
The second file,
installation.configuration.preferences
is shown below:
This file tells JMP Clinical where to find
installation.path.preferences
file for each of the locations configured for your JMP Clinical installation.
Note
: There is a separate
installation.path.preferences
file for each local and shared configuration for JMP Clinical. Conversely, there is only one
installation.configuration.preferences
file.
To set up a shared location for your studies, you must first map a location on a shared drive and then modify the
installation.configuration.preferences
file that is installed with JMP Clinical.
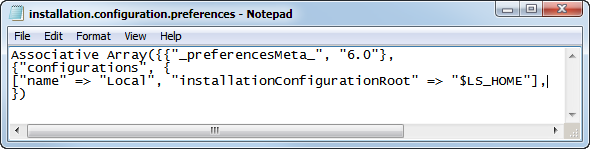
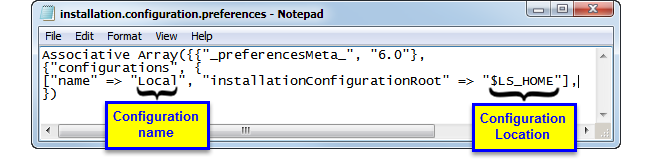
Examine the
installation.configuration.file
shown below:
[
“name” => “Local”, “installationconfigurationroot” => “$LS_HOME”]
This line defines both the name and location of the default configuration. In this case, the name of the configuration is
Local
and it is located in the
$LS_HOME
, which in the default installation is set to
C:\Program Files\SASHome\JMPClinical\12\LifeSciences\.
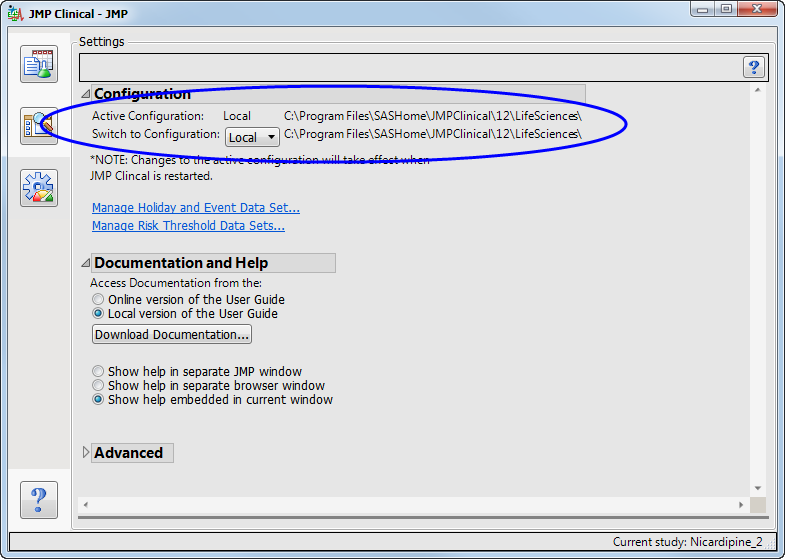
This is the location shown on the JMP Clinical
Settings
tab, as shown below:
|
|
Navigate to the JMP Clinical installation folder (normally
C:\Program Files\SASHome\JMPClinical\12\LifeSciences
) on your local machine and open the
installation.configuration.preferences
file with a text editor.
|
[
“name” => “Local”, “installationconfigurationroot” => “$LS_HOME”],
[
“name” => “Local”, “installationconfigurationroot” => “$LS_HOME”]
|
|
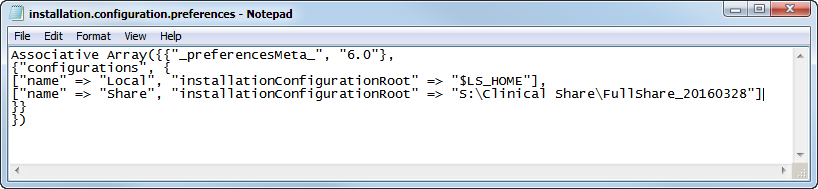
Change the name and location in the pasted copy to the name and location of the share. In this case the name is
Share
and the location is
S:\Clinical Share\FullShare_20160328
. Your name and location must be enclosed by the quotation marks. The modified statement should appear as shown below:
|
[
“name” => “Share”, “installationconfigurationroot” => “S:\Clinical Share\FullShare_20160328”]
Note
: Entries are case-sensitive.
Caution
: In the example shown here, the location is specified using a mapped drive path. Locations should contain mapped drive paths only if your organization's setup guarantees that all users have the same mapped drives. If you are unsure, you should use the UNC path instead.
|
|
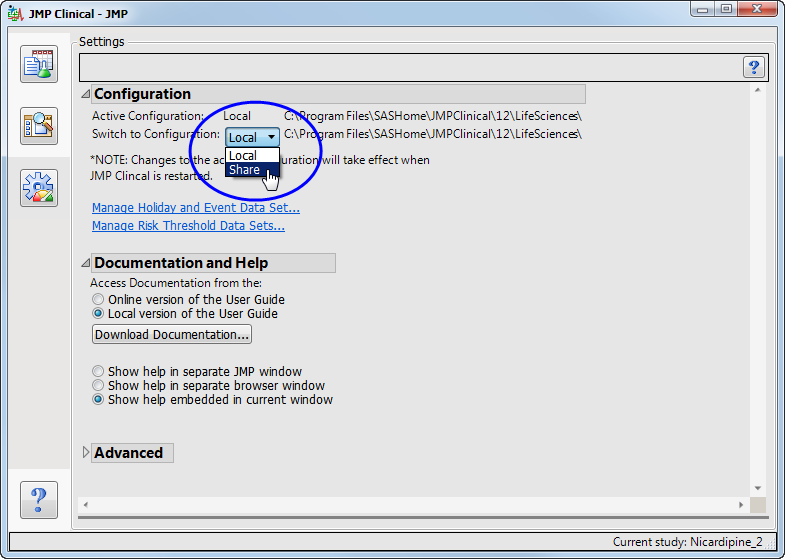
The new “
Share
” configuration is now shown as an option.
|
|
Select
Share
.
|
You must close and reopen JMP Clinical before the new selection takes effect. If the new Share folder is an existing shared folder, studies contained within the folder are displayed on the
Studies
tab. If this is a new shared folder, JMP Clinical will add the necessary components for the folder to function as a shared studies folder.
Note
: Anyone with Read/Write privileges on the shared drive will have access to a new shared folder. As the creator of this folder you can control level of access by changing the permissions using WinO/S commands.
|
•
|
When a partial date identified (
xxDTC
for
LB
,
EG
or
VS
), an asterisk (*) is appended to the end of the finding name or test code. You should review the findings for the appropriately reported set of observations.
|
|
•
|
When the reference date (
RFSTDTC
) is partial, an asterisk is appended to the
AETERM
. You should review all reported dates, study days, and contents for correctness.
|
|
•
|
When the AE start date (
AESTDTC
) is partial, an asterisk is appended to the date in the narrative. You should review all contents of the narrative.
|
|
•
|
When the AE end date (
AEENDTC
) is partial, an asterisk is appended to the date in the narrative. You should review the final outcome and narrative header information for correctness.
|
|
•
|
When any of the dosing records have partial dates for
exstdtc
or
exendtc
, an asterisk is placed in the drug header that explains dose at time of the event, or the pre- or post-dose status. All text related to the drug should be reviewed.
|
|
•
|
When the date of completion or discontinuation (
DSSTDTC
) is partial, an asterisk is appended to the date in the narrative. You should review these dates for correctness.
|
|
•
|
When either or both of the start or stop dates (
CMSTDTC
or
CMENDTC
) for Concomitant medications are partial, an asterisk is appended to the end of
CMTERM
or
CMDECOD
(based on the selected analysis option). You should review the data for this medication for correctness.
|
When running Findings reports,
JMP Clinical
looks for and appends the values from either
xxPOS or xxSPEC
to the test names in
xxTESTCD
and
xxTEST
. This enables you to analyze findings data when multiple findings test names are identical across the variables:
xxTESTCD
,
xxPOS
, and
xxSPEC
.
If test name values are still not unique across categories of
xxCAT
or
xxSCAT
(if they exist) after appending the prior variables, a numeric index is appended to non-unique tests so that reports can still be run and tests are not inappropriately combined.
How does JMP Clinical define various terms for risk-based monitoring?
1
Subjects are considered
RANDOMIZED
if there is at least one record from
DS
where the index ((
DS.DSDECOD
2
),
RANDOMIZED
) is true
Depending on the available information, subjects are considered
SCREEN FAILURES
if:
|
•
|
|
•
|
the values in
DS.EPOCH
is
SCREENING
, the value in
DS.DSCAT
is
DISPOSITION EVENT,
and value in
DS.DECOD
is
COMPLETED
, or
|
|
•
|
To determine whether the subjects have
COMPLETED
the trial, a
The SAS WHERE Expression
can be included on the analysis dialog to select the appropriate
DS
records (this statement should also select the records that indicate whether a subject has alternatively
DISCONTINUED
or
WITHDRAWN
). If this
The SAS WHERE Expression
is supplied and the value in
DS.DSDECOD
is
COMPLETED
, the subject is considered to have completed the trial. Otherwise, based on the available variables, the subject is considered to have completed the trial only if
|
•
|
the value in
DS.EPOCH
is
TREATMENT
and the value in
DS.DSCAT
is
DISPOSITION EVENT
and the value in
DS.DSDECOD
is
COMPLETED
, or
|
|
•
|
|
•
|
Subjects are considered to have
DISCONTINUED
or
WITHDRAWN
when a
The SAS WHERE Expression
is supplied and
DS.DSDECOD
is
^=COMPLETED
. Otherwise, based on the available variables the subject is considered to have discontinued the trial if
|
•
|
the value in
DS.EPOCH
is
TREATMENT
and the value in
DS.DSCAT
is
DISPOSITION EVENT
and the value in
DS.DSDECOD
is
^= COMPLETED
, or
|
|
•
|
|
•
|
|
•
|
Date/Time of First Study Treatment (
DM.RFXSTDTC
) is nonmissing, or
|
|
•
|
If records are available for the subject in the
EX
domain, or
|
|
•
|
|
•
|
An AE is considered fatal if the value in either
AE.AEOUT
or is either FATAL or DEATH, or if the value in
AESDTH
is either
Y
or
YES
.
A subject is considered to have signed informed consent if there is a value for Date/Time of Informed Consent (
DM.RFICDTC
).
|
•
|
the value in Date/Time of Death (
DM.DTHDTC
) is nonmissing, or
|
|
•
|
|
•
|
the subject discontinued the trial with and the value in
DS.DSDECOD
is either
DEATH
or
DIED
or
DEAD
, or
|
|
•
|
|
•
|
If the value in
DS.DSDECOD
is either
DEATH
,
DIED
, or
DEAD
, then the subject is considered to have
Discontinued Due to Death
, or
|
|
•
|
|
•
|
If the value in
DS.DSDECOD
is either
LOST TO FOLLOW-UP
,
LOST TO FOLLOWUP
,
LOST TO FOLLOW UP
, or
LTFU
, then the subject is considered to be
Lost to Followup
, or
|
|
•
|
If the value in
DS.DSDECOD
is either
ADVERSE EVENT
or
AE
, then the subject is considered to have
Discontinued Due to Adverse Event
, or
|
|
•
|
If the value in
DS.DSDECOD
is either WITHDRAWAL BY SUBJECT,
SUBJECT WITHDRAWAL
,
WITHDREW CONSENT
, or
SUBJECT WITHDREW CONSENT
, then the
Patient Withdrew from Study
, or
|
|
•
|
If none of the conditions listed above apply, the subject is considered to have
Discontinued for Other Reasons
.
|
where
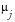 is the
mean
,
median
or user-supplied
center value
. The quantity
is the
mean
,
median
or user-supplied
center value
. The quantity
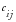 equals
equals
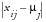 ,
,
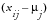 , and
, and
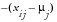 , for
Direction of Risk Signals
equal to B, U, and L, respectively, and
, for
Direction of Risk Signals
equal to B, U, and L, respectively, and
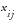 is the value for the
i
t
h
site or country and the
j
th
risk indicator.
is the value for the
i
t
h
site or country and the
j
th
risk indicator.
It is acceptable to specify both yellow and red risk thresholds, one or no risk thresholds. When specifying only a moderate threshold, the
Red Percent of Center
is left missing in the risk threshold data set so that moderate risk is considered
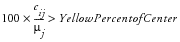 . In instances where values do not meet the criteria for moderate or severe risk, the risk is considered mild (green). Note that for risk thresholds defined using the above criteria, no threshold colors are determined in instances where the
mean
,
median
or
center value
is calculated or set to zero.
. In instances where values do not meet the criteria for moderate or severe risk, the risk is considered mild (green). Note that for risk thresholds defined using the above criteria, no threshold colors are determined in instances where the
mean
,
median
or
center value
is calculated or set to zero.
In this case, it is acceptable to specify both thresholds, one threshold, or no risk thresholds at all. When specifying only a moderate threshold, the
Red Magnitude
is left missing in the risk threshold data set so that moderate risk is
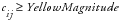 . In cases where neither moderate nor severe risk applies, the risk is considered mild (green).
. In cases where neither moderate nor severe risk applies, the risk is considered mild (green).
There are five overall risk indicators. These are either weighted averages or combinations of the individual risk indicators for which at least one risk threshold is defined, where both the
Weight for Overall Risk Indicator
and the
standard deviation
of the indicator > 0.
The first, or
Overall Risk Indicator
, incorporates all of the variables meeting these criteria into a single measure that signifies the overall risk and performance of a clinical site. This indicator is generated only when the
Weight for Overall Risk Indicator
exceeds 0 for at least one of the available risk indicators exhibiting variability. If none of the individual indicators have a
Weight for Overall Risk Indicator > 0,
then the corresponding
Overall Risk Indicator
is not generated.
Each of the other four overall indicators -
Enrollment Metrics
,
Disposition
,
Adverse Events
, and
Manually Entered
- combines subsets of the risk indicators based on
Category
in the risk weight data set. By default,
Category
matches how variables are grouped in Risk-Based Monitoring, with
Manually Entered
applied to all user-supplied risk indicators from
Update Study Risk Data Set
. If no indicators have a
Weight for Overall Risk Indicator
> 0 for a given category, then the corresponding overall indicator is not provided.
The
Weight for Overall Risk Indicator
(
w
j
) can either be missing (in this case, it is assumed to be zero) or greater than or equal to zero. The weights are self-normalizing in that each weight is divided by the sum of all weights for variables contributing to the particular overall indicator. The contribution of each indicator to an overall indicator is based on its weight, center value (either
mean
,
median
or user-provided
center value
,
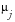 ), standard deviation (
), standard deviation (
 ), and direction. In general, the value for an overall indicator for the
i
th
site or country and the
j
th
risk indicator is defined as
), and direction. In general, the value for an overall indicator for the
i
th
site or country and the
j
th
risk indicator is defined as
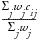 , where
, where
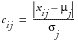 ,
,
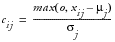 , or
, or
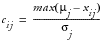 when Direction equals B,U, or L, respectively. This can be interpreted as larger values imply greater risk. By default, all weights are assumed equal to one in the Default Risk Threshold data set, meaning that each variable contributes equally to each overall indicator.
when Direction equals B,U, or L, respectively. This can be interpreted as larger values imply greater risk. By default, all weights are assumed equal to one in the Default Risk Threshold data set, meaning that each variable contributes equally to each overall indicator.
To avoid confusion in this FAQ, variables are written as
domain.domain-variable
. For example, USUBJID from the DM domain is written as
DM.USUBJID
. When a term can be applied to multiple domains, "xx" is used to imply a two-letter domain code.
Note: Although variables are listed in uppercase in this document, JMP Clinical is case insensitive.