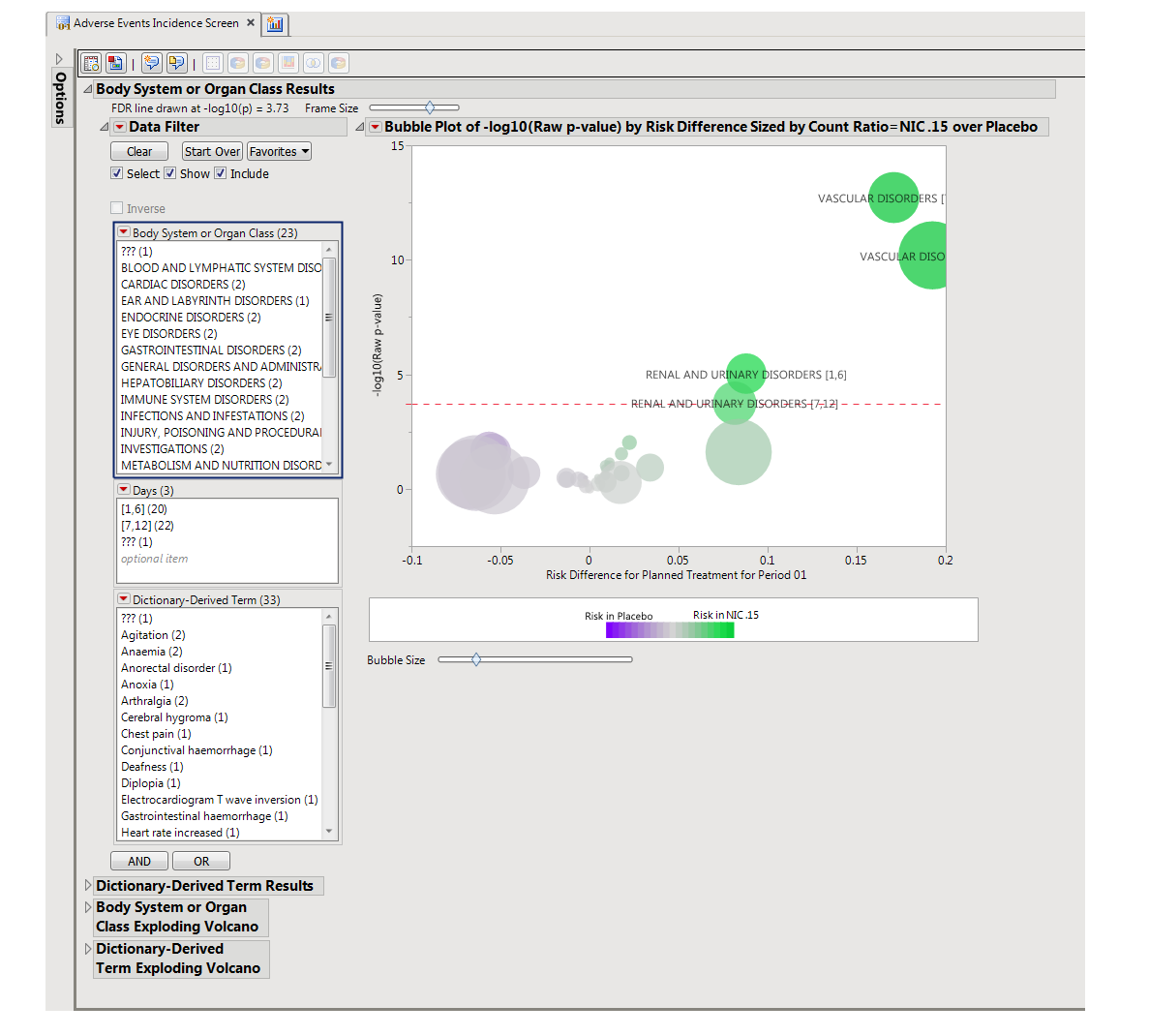
The AE Incidence Screen report screens all adverse events by performing a Cochran-Mantel-Haenszel exact test on all 2 x 2 tables constructed from event incidence and treatment arm. Output one or more volcano plots of risk differences when using default settings. However, plots of relative risks or odds ratios can be generated depending on the selected option for the x-Axis for Volcano Plot. If Study ID varies among the subjects for analysis, the test is stratified by Study ID.
|
•
|
Perform Double FDR Adjustment checked,
|
|
•
|
Group Level set to Body System or Organ Class, and
|
|
•
|
Trial Time Windows of [1,6][7,12]
|
generates the report shown below. Refer to the AE Incidence Screen requirements description for more information.
The Report contains the following sections:
|
•
|
Body System or Organ Class Results: This tab presents a data filter and a Volcano Plot with the adverse events summarized at the selected Group Level. Note that the name of this tab reflects whatever term is selected as Group Level and is presented only if Perform Double FDR Adjustment is selected.
|
|
•
|
Dictionary-Derived Term Results: This tab contains a data filter and a Bubble Plot for each event defined at the Term Level for each statistically significant group and time window (if present) that appears on the Body System or Organ Class Results tab if Perform Double FDR Adjustment is checked. If Perform Double FDR Adjustment is not checked, all adverse events at the Term Level are presented. Note that the name of this tab reflects whatever term is selected as Term Level.
|
|
•
|
Body System or Organ Class Exploding Volcano: Contains a data filter and an animation across the time windows of the results included on the Body System or Organ Class Results tab. Note that the name of this tab reflects whatever is selected as Group Level and is presented only if Trial Time Windows are provided and Perform Double FDR Adjustment is selected.
|
|
•
|
Dictionary-Derived Term Exploding Volcano: Contains a data filter and an animation across the time windows of the results included on the Dictionary-Derived Term Results tab, which presents a separate bubble for each term or time window. Note that the name of this tab reflects whatever term is selected as Term Level and is presented only if Trial Time Windows are provided.
|
|
•
|
Dot Plot: Click
|
|
•
|
Relative Risk Plot: Click
|
|
•
|
Odds Ratio Plot: Click
|
|
•
|
Contingency Analysis: Click
|
|
•
|
Venn Diagram: Click
|
|
•
|
Tabulate: Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the arrow to reopen the completed process dialog used to generate this output.
|
Note: For information about how treatment emergent adverse events (TEAEs) are defined in JMP Clinical, please refer to Determining If an Event Is a Treatment Emergent Adverse Event.