This report creates tabular and graphical overviews of treatment emergent
adverse events
for the safety
population
by actual treatment
arm
.
Running
Treatment Emergent AE Summary
for
Nicardipine
using default settings generates the
Report
shown below.
|
•
|
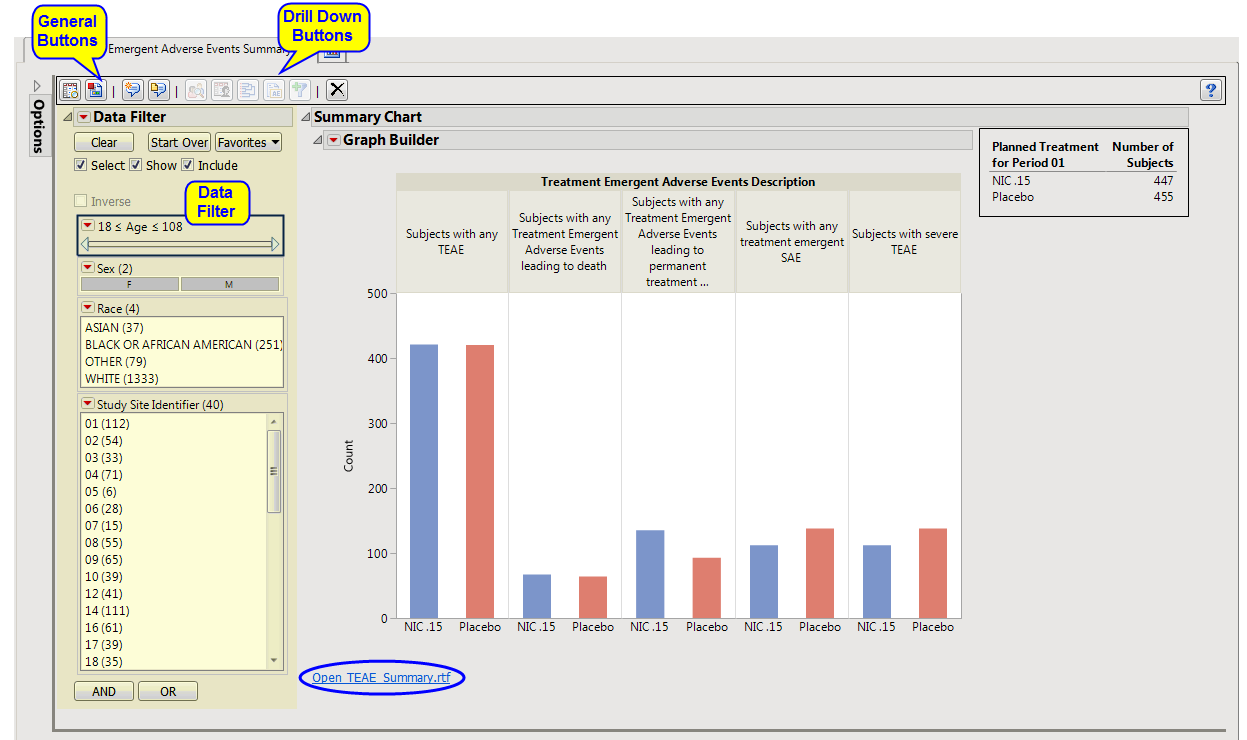
Summary Chart
: This section contains a
Bar Chart
summarizing the counts of various treatment emergent
adverse events
by treatment. Click the
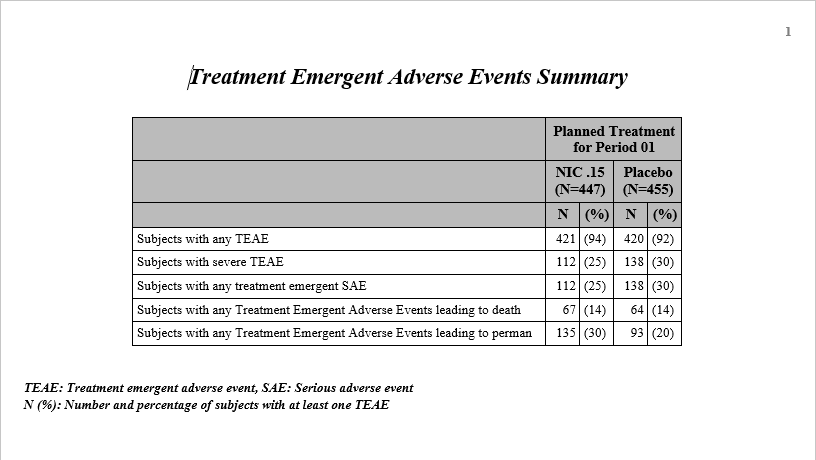
Open TEAE Summary.rtf
link to open an rtf file (shown below) that summarizes the treatment emergent adverse events.
Percentages
of subjects experiencing an event by
arm
are also included in this summary.
|
This enables you to subset subjects based on demographic characteristics and study site. Refer to
Data Filter
for more information.
|
•
|
Profile Subjects
: Select subjects and click
|
|
•
|
Show Subjects
: Select subjects and click
|
|
•
|
Cluster Subjects
: Select subjects and click
|
|
•
|
AE Narrative
: Select subjects and click
|
|
•
|
Demographic Counts
: Select subjects and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the
arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the
Options
tab to open a dynamic report navigator that lists all of the reports in the review. Refer to
Report Navigator
for more information.
|
Note
: For information about how treatment emergent adverse events (TEAEs) are defined in JMP Clinical, please refer to
How does JMP Clinical determine whether an Event Is a Treatment Emergent Adverse Event?
.
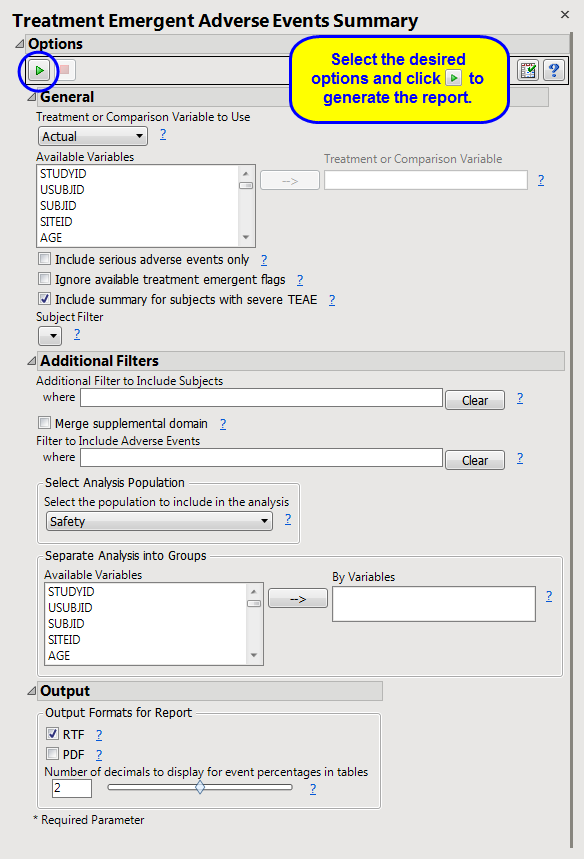
Include serious adverse events only
,
Ignore available treatment emergent flags
,
Include summary for subjects with severe TEAE
Additional Filter to Include Subjects
2
,
Merge supplemental domain
,
Filter to Include Adverse Events
,
Select the population to include in the analysis
,
By Variables
Subject-specific filters must be created using the
Create Subject Filter
report prior to your analysis.
For more information about how to specify a filter using this option, see
The SAS WHERE Expression
.