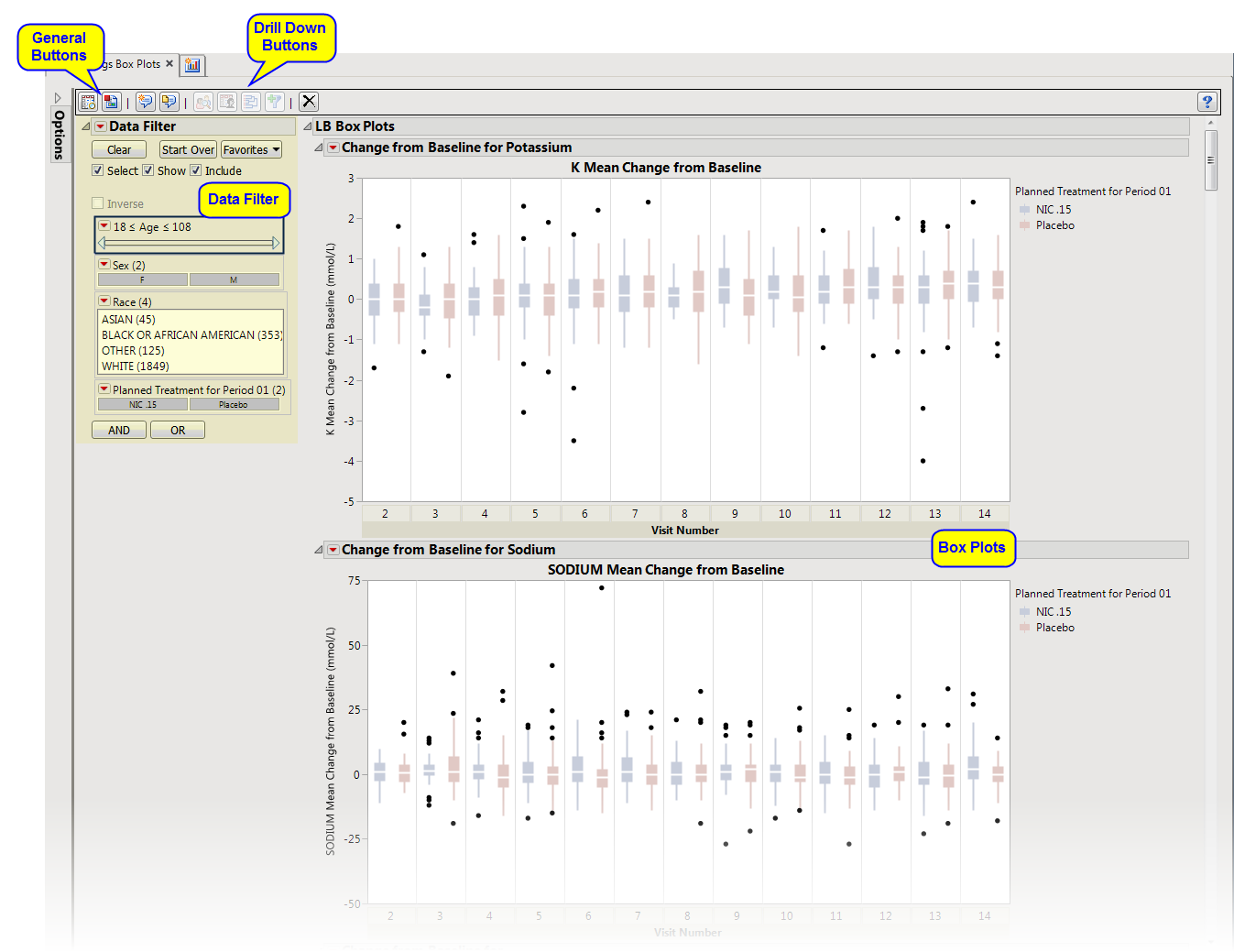
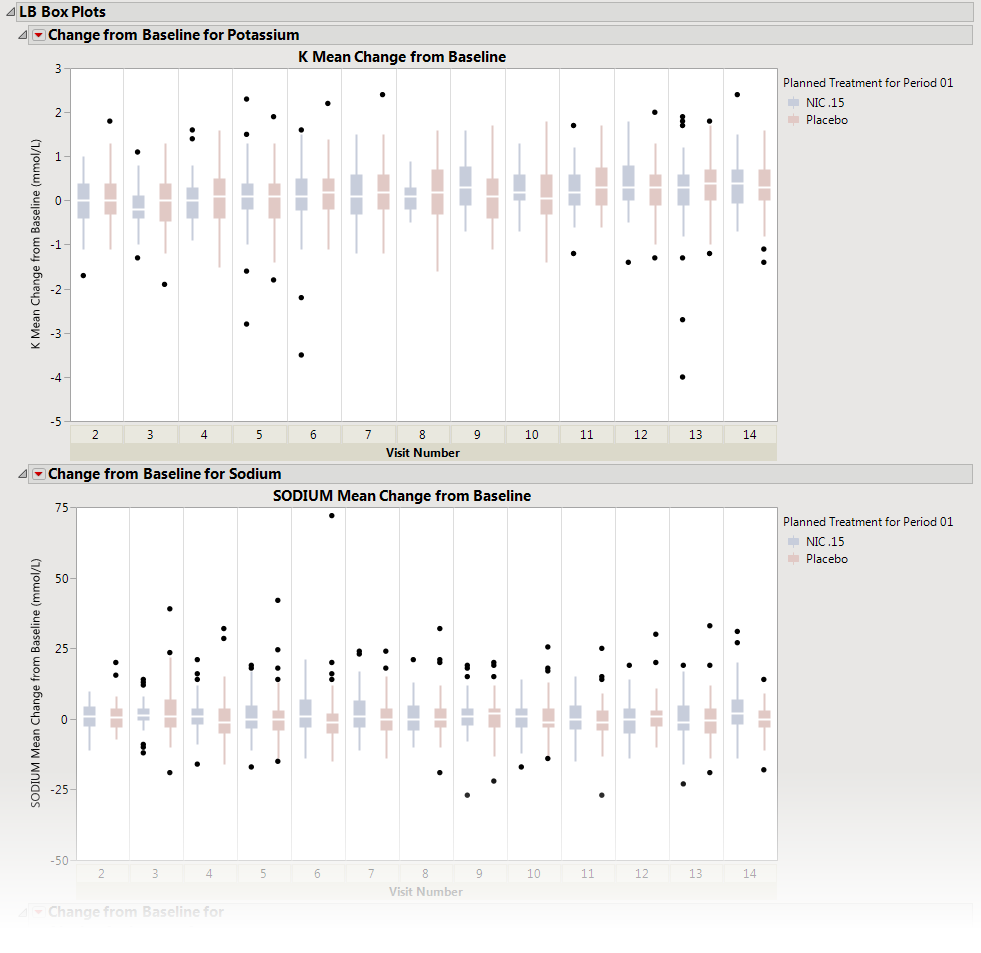
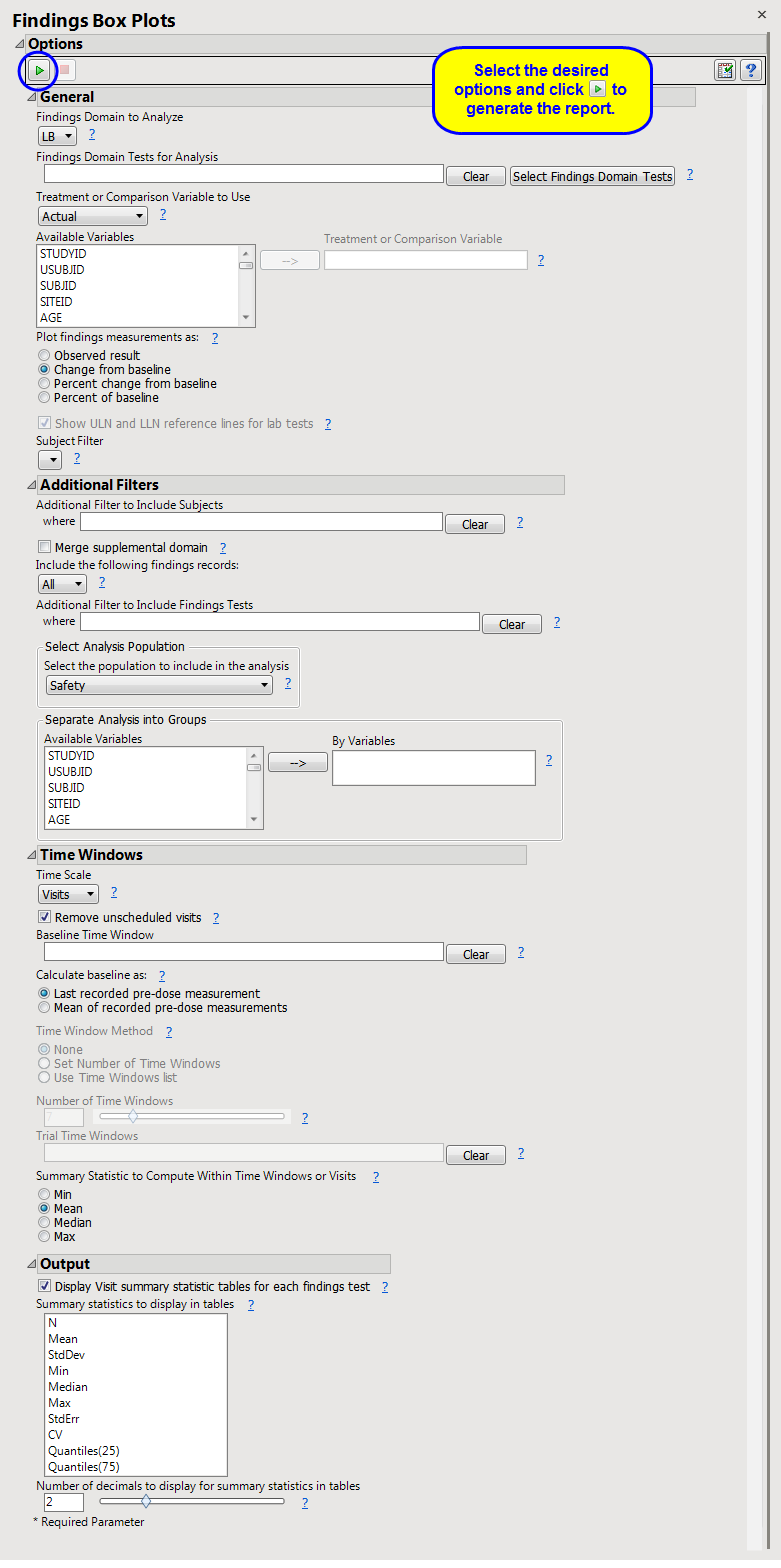
This report displays the
box plots
by treatment group representing the change from baseline in measurements for each test for specified findings domain across various time s or points in the study. Time s can be specified using a list of bracketed times or, alternatively, a number of time s can be set to create time s that span across the entire study.
If the
variable
xxBLCHG
(where
xx
is substituted with the chosen domain 2 letter code) exists, this variable is used in plotting change from baseline. Otherwise, a measurement is determined to be a baseline measurement by the
ABLFL
or
xxBLFL
variable where
xx
is substituted with the 2-letter code for the chosen domain for analysis. If this variable does not exist, baseline is calculated from measurements taken on or before day 1 of the study. A time can be specified to determine baseline measurements.
Note
: JMP Clinical uses a special protocol for data including non-unique Findings test names. Refer to
How does JMP Clinical handle non-unique Findings test names?
for more information.
Running
Findings Box Plots
for
Nicardipine
using default settings generates the report shown below. Output from the report is organized into sections. Each section contains one or more plots, data panels, data filters, or other elements that facilitate your analysis.
Presents several
Box Plot
s
representing the values chosen in
Plot findings measurements as:
from the
dialog
. The name of this section varies based on the domain chosen.
Note
: The name of this section and the findings results (
LB
,
VS
, or
EG
) displayed depend on the domain selected using the
Findings Domain to Analyze
option.
Each
box plot
set represents an
xxTESTCD
from the selected domain. For example, in the graph above, the first figure represents
K
(Potassium). The first set of boxes (
Trial Max
) represents change from baseline versus the maximum Potassium value that occurs in the study. The second set of boxes (
Trial Mean
) represents change from baseline versus the average Potassium value that occurs in the study. The third set of boxes (
Trial Median
) represents change from baseline versus the
median
Potassium value that occurs in the study. The final set of boxes (
Trial Min
) represents change from baseline versus the minimum Potassium value that occurs in the study.
This enables you to subset subjects based on demographic characteristics and other factors. Refer to
Data Filter
for more information.
|
•
|
Profile Subjects
: Select subjects and click
|
|
•
|
Show Subjects
: Select subjects and click
|
|
•
|
Cluster Subjects
: Select subjects and click
|
|
•
|
Demographic Counts
: Select subjects and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the
arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the
Options
tab to open a dynamic report navigator that lists all of the reports in the review. Refer to
Report Navigator
for more information.
|
Additional Filter to Include Subjects
2
,
Merge supplemental domain
,
Include the following findings records:
,
Additional Filter to Include Findings Tests
,
Select the population to include in the analysis
,
By Variables
Time Scale
,
Remove unscheduled visits
,
Baseline Time Window
,
Calculate baseline as:
,
Time Window Method
,
Number of Time Windows
, ,
Trial Time Windows
,
Summary Statistic to Compute Within Time Windows or Visits
Display Visit summary statistic tables for each findings test
,
Summary Statistics to Display in Tables
,
Number of decimals to display for summary statistics in tables
Subject-specific filters must be created using the
Create Subject Filter
report prior to your analysis.
For more information about how to specify a filter using this option, see
The SAS WHERE Expression
.