Note
: JMP Clinical uses a special protocol for data including non-unique Findings test names. Refer to
How does JMP Clinical handle non-unique Findings test names?
for more information.
Running Findings Time to Event for
Nicardipine
generates the
Report
shown below. Refer to the
Findings Time to Event
requirements description for more information.
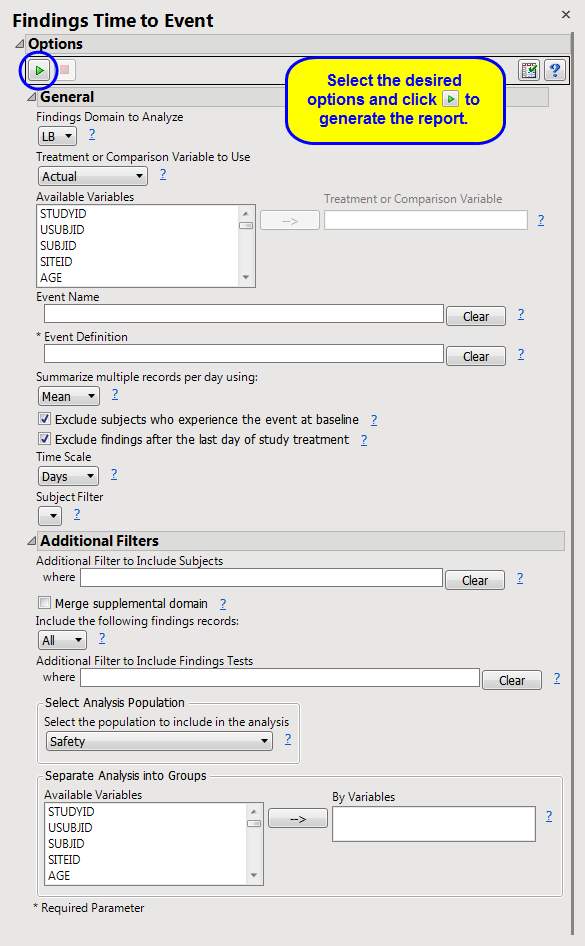
Note
: In this example,
VS
was selected as the
Findings Domain to Analyze
,
Hypertension
was specified as the
Event Name
, and
SYSBP >= 140 and DIABP >= 90
was entered as the
Event Definition
.
|
•
|
The name of this report corresponds to the
Event Name
supplied in the
dialog
. If this is
not
supplied, it is called
Domain Event
where
Domain
is determined by the
Findings Domain to Analyze
selected in the dialog. For example, if the event is defined using the
VS
domain, the section is called
VS Event
. This section contains a
Kaplan-Meier
analysis of the time to the defined event.
|
This analysis compares the time until the event among treatments in the study. Subjects
not
experiencing the defined event are
censored
at the date of their last available findings data.
See
Survival Plot
for more information.
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the
arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the
Options
tab to open a dynamic report navigator that lists all of the reports in the review. Refer to
Report Navigator
for more information.
|
Findings Domain to Analyze
,
Treatment or Comparison Variable to Use
,
Treatment or Comparison Variable
Exclude subjects who experience the event at baseline
,
Exclude findings after the last day of study treatment
Additional Filter to Include Subjects
2
,
Merge supplemental domain
,
Include the following findings records:
,
Additional Filter to Include Findings Tests
,
Select the population to include in the analysis
,
By Variables
Subject-specific filters must be created using the
Create Subject Filter
report prior to your analysis.
For more information about how to specify a filter using this option, see
The SAS WHERE Expression
.