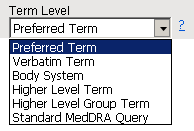
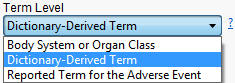
Use this option to specify which level of terms (for example,
adverse event
,
intervention
, or
MedDRA
) to use as categories.
|
AEDECOD
in
AE
|
|
|||||
|
AETERM
in
AE
|
|
|||||
|
AEBODSYS
in
AE
|
|
|||||
|
AEHLT
in
AE
|
||||||
|
AEHLGT
in
AE
|
||||||
|
AESOC
in
AE
|
|
|||||
|
AELLT
in
AE
|
|
|||||
|
AEBODSYS
in
AE
|
||||||
|
AEDECOD
in
AE
|
||||||
|
AETERM
in
AE
|
||||||
Note
: The dictionary name and version used to generate the descriptions should be included as part of the submission.