|
•
|
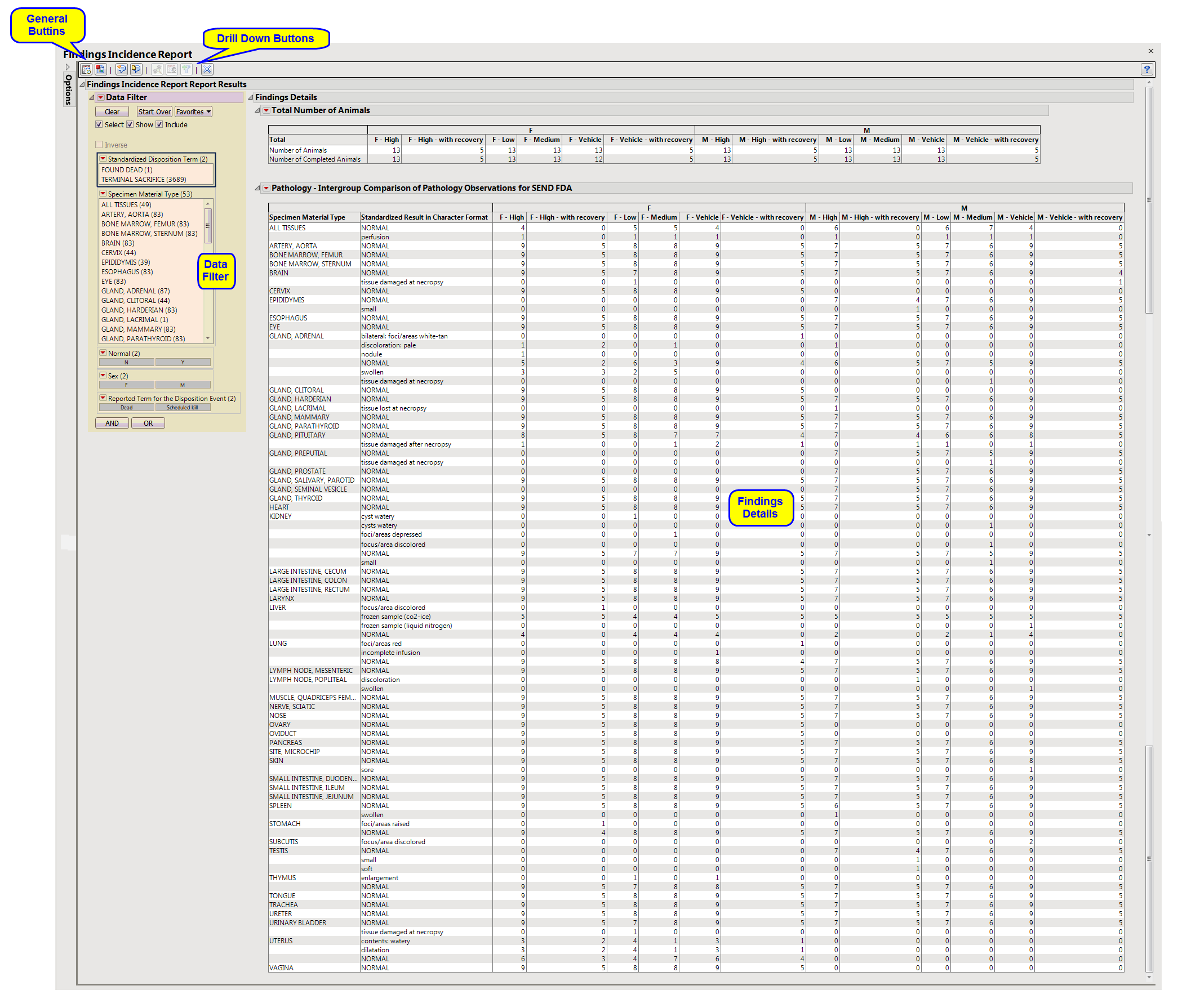
Findings Details: Lists two tables providing details about the number, demographics and distribution of animals among the treatment groups and a high-level summary of the findings.
|
|
•
|
Data Filter: This enables you to subset subjects based on demographic characteristics, disposition, tissue, and other factors. Refer to Data Filter for more information.
|
|
•
|
Profile Animals: Generates a static report showing the profile(s) for the selected animals. You must first select one or more animals from either the Intergroup Comparison of Pathology Operations table or the Specimen Type Pathology Comparison table and then click
|
Note: You must click  to view the associated data tables, including the Specimen Type Pathology Comparison table.
to view the associated data tables, including the Specimen Type Pathology Comparison table.
|
•
|
Show Animals: Select groups of animals from the Intergroup Comparison of Pathology Operations table or rows from the Specimen Type Pathology Comparison table and click
|
|
•
|
Create Animal Filter: Click to create a filter that can be applied in subsequent analyses to reduce the analyzed animals to only the ones currently selected.
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information.
|