This report generates an exposure plot for all subjects in a study for an investigational product by dose and exposure time for the safety population by treatment. Summaries of exposure duration for subjects are also produced along with a static report of counts of total duration periods for subjects. The duration report and summary can be produced for specific duration times only through use of a duration time windows list on the Exposure Duration tab. The exposure plot is generated for all subjects across any time of exposure.
The Report contains the following elements:
|
•
|
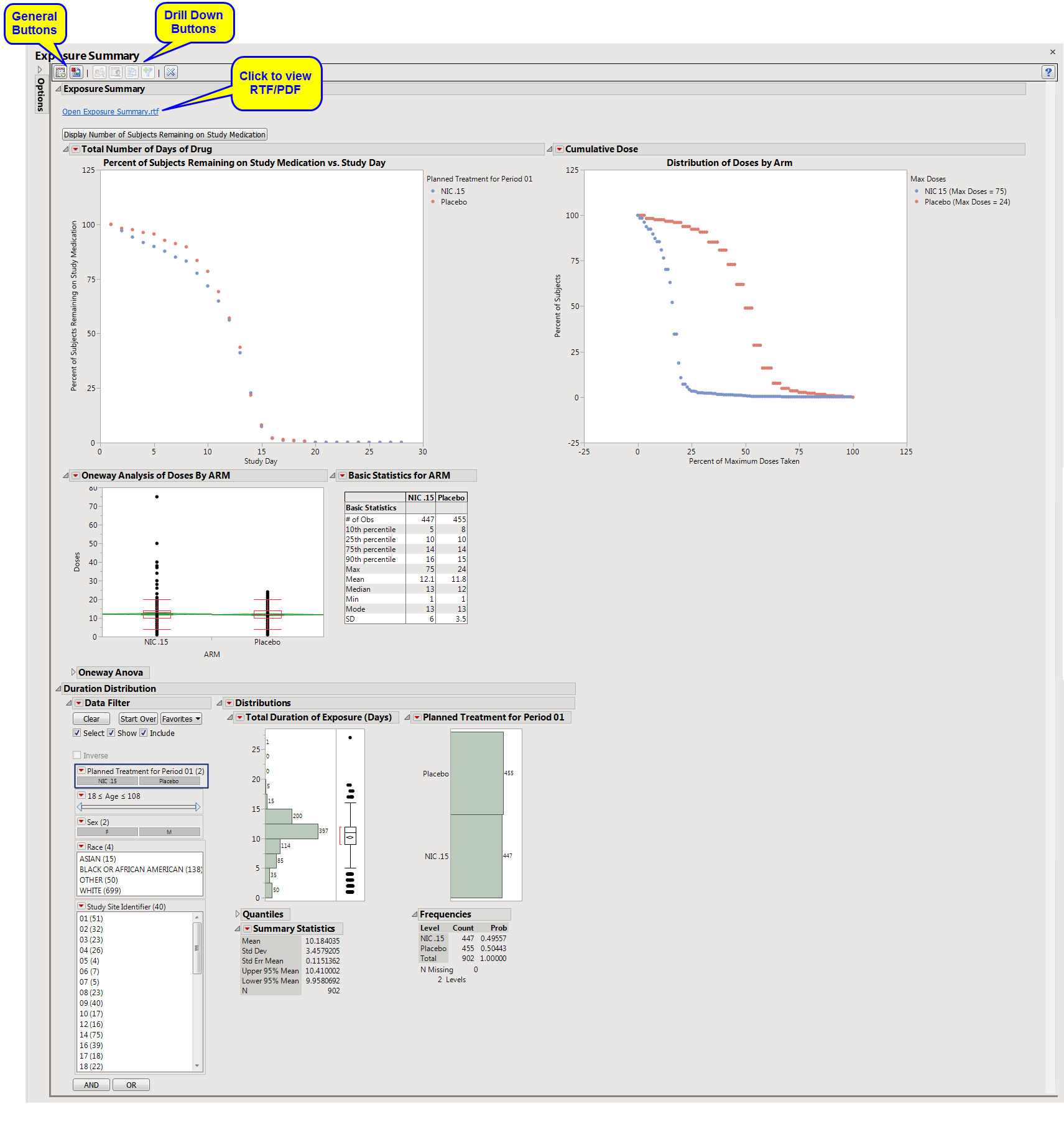
Exposure Summary: Displays three plots showing the percent of subjects who remain on the study medication by Day of Study, the distribution of doses by arm, and a list of dose descriptive statistics by ARM along with a Box Plot of doses.
|
|
•
|
Duration Distribution: Presents a Histogram for the total duration of exposure in terms of the number of days exposed to treatment.
|
Note: You can toggle between viewing percentage or number of subjects by clicking .
|
•
|
ThProfile Subjects: Select subjects and click
|
|
•
|
Show Subjects: Select subjects and click
|
|
•
|
Cluster Subjects: Select subjects and click
|
|
•
|
Demographic Counts: Select subjects and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the arrow to reopen the completed dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information.
|
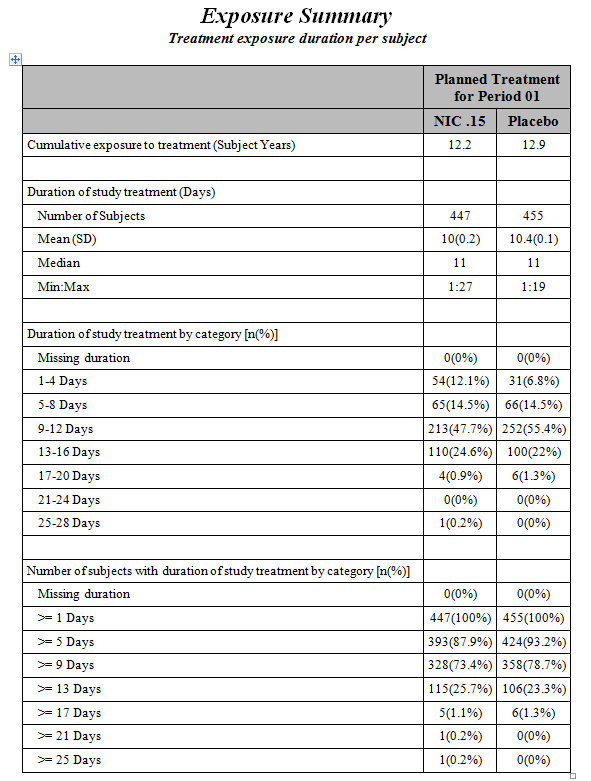
An Exposure Summary Report is also generated. Click the Open Exposure Summary link to view this report. It summarizes the dosing in terms of Subject Years, presents summary statistics of subjects’ exposure to treatment, the frequency of subjects within exposure categories (number and range determined by Number of Duration Time Windows and Duration Time Windows in the dialog), and the cumulative frequency within exposure categories. If Exposure Duration (EXDUR) is missing and end date/time of treatment (EXENDT) is incomplete or missing for a subject(s), the subject(s) are excluded from the min/max duration times in the report.
|
•
|
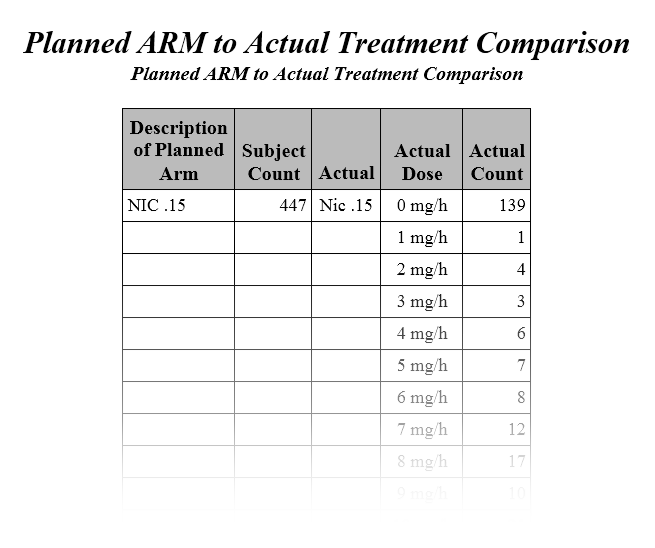
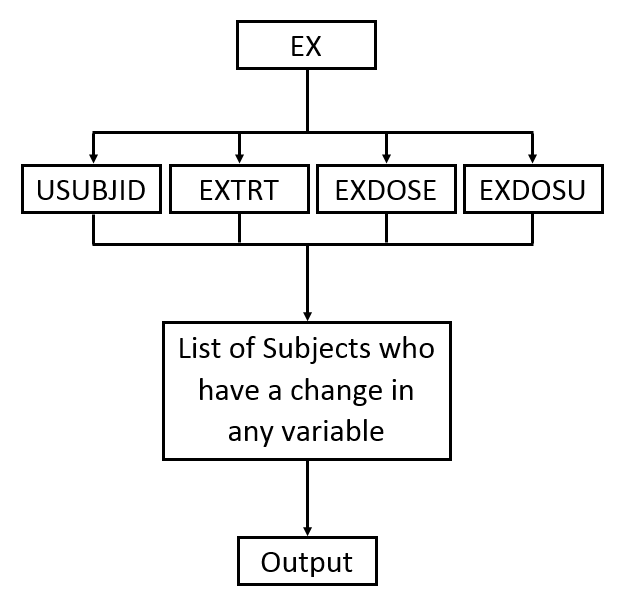
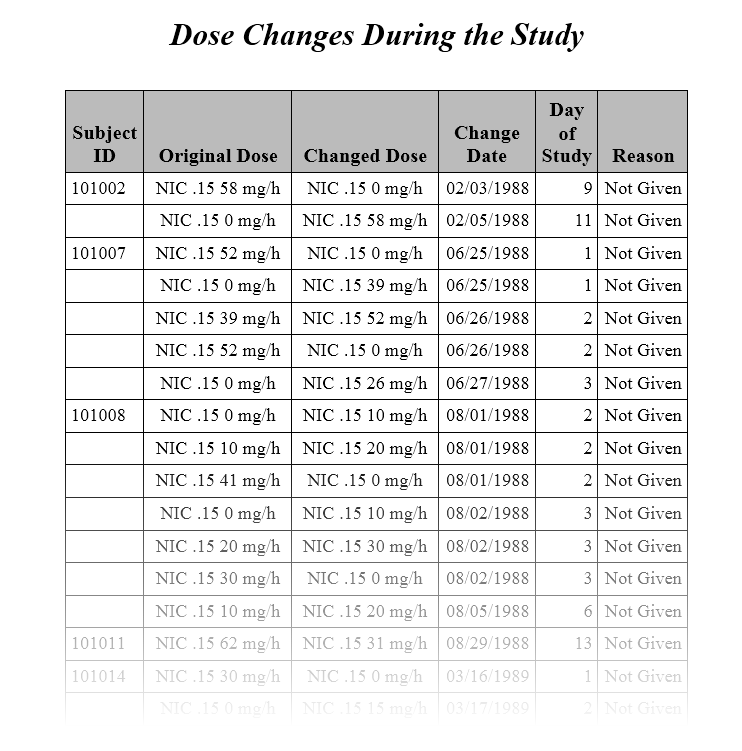
The second Table (shown below) lists the changes in dosage each subject received over the course of the study. JMP Clinical uses data from USUBJID, EXTRT, EXDOSE, and EXDOSU to generate the list of subjects with dosage changes reported in the table (as shown in the diagram below).
|
The original dose and the changed dose are calculated using combined data from EXTRT/EXDOSE/ EXDOSU. The date and reason the dosage was changed are provided by EXENDTC and EXADJ. The default response of Not Given is shown whenever EXADJ is either empty or non-existent.