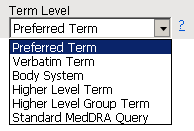
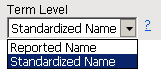
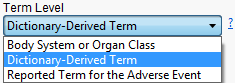
Use this option to specify which level of terms (for example, adverse event, intervention, or MedDRA) to use as categories.
|
AEDECOD in AE
|
|
|||||
|
AETERM in AE
|
|
|||||
|
AEBODSYS in AE
|
|
|||||
|
AEHLT in AE
|
||||||
|
AEHLGT in AE
|
||||||
|
AESOC in AE
|
|
|||||
|
AELLT in AE
|
|
|||||
|
AEBODSYS in AE
|
||||||
|
AEDECOD in AE
|
||||||
|
AETERM in AE
|
||||||
Note: The dictionary name and version used to generate the descriptions should be included as part of the submission.