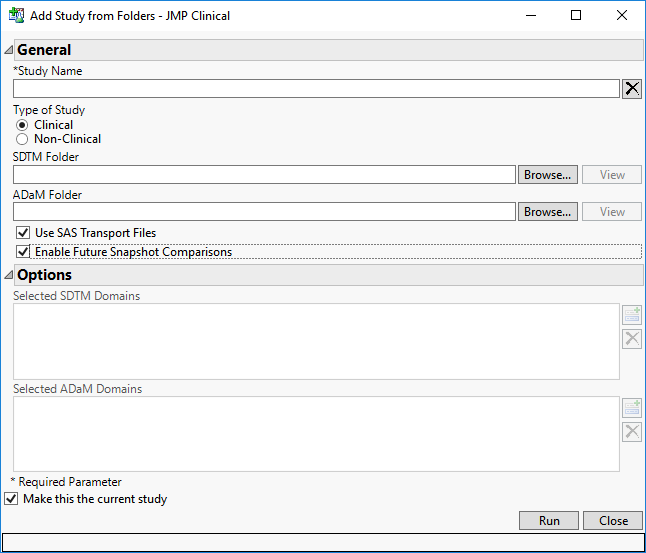
This report adds a study (see Studies) from either local folders or a SAS Drug Development (SDD) server that contain
CDISC-formatted data. You must add a study before you can perform the analysis. This report can also import
SAS transport files for you.
The study and associated metadata are added to your database.
Note: If your JMP Clinical software has been configured for SAS Drug Development, clicking

expands a drop-down menu. Select
From Folders from the drop-down menu to open the
Add Study dialog.
Note: When adding a clinical study and assigning both ADAM and SDTM domains, if you do not explicitly specify which SDTM and ADAM domains to include, the ADAM equivalent of each domain is added and the and the corresponding SDTM data set excluded. For example, if both
ADAE and
AE are present, then
ADAE takes precedence and is included, whereas AE is not included.
 expands a drop-down menu. Select From Folders from the drop-down menu to open the Add Study dialog.
expands a drop-down menu. Select From Folders from the drop-down menu to open the Add Study dialog.