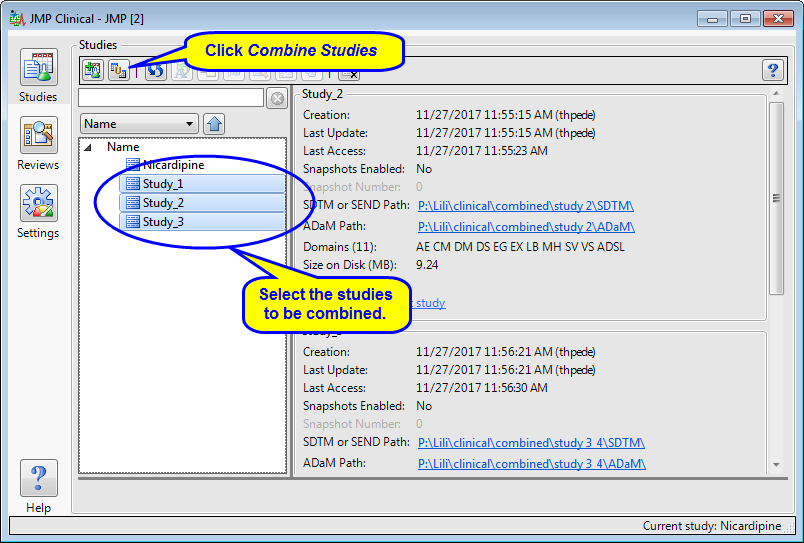
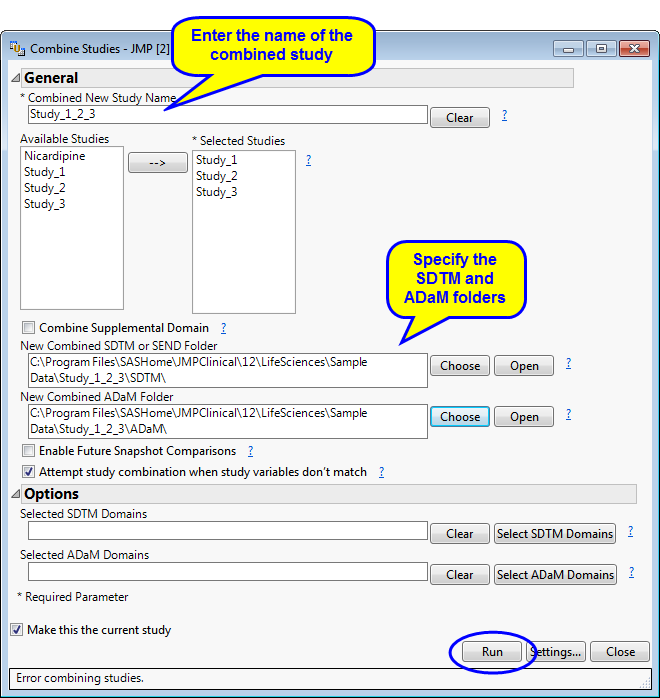
Running this report preserves all original data from studies to be combined, and creates new input data sets by appending rows from each corresponding parent data set. The USUBJID values of the new input data sets contain both the parent study name and USUBJID value. The new input data sets are then used to add the combined study to JMP Clinical.
|
|||||||||||
|
|||||||||||
|
|||||||||||
|
|||||||||||
|
|||||||||||
|
Note: In cases where there are variables that cannot be combined, this report generates a report listing those variables.
|
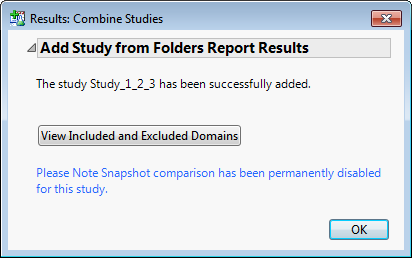
When the combination process is complete, the following Results window appears.
|
|
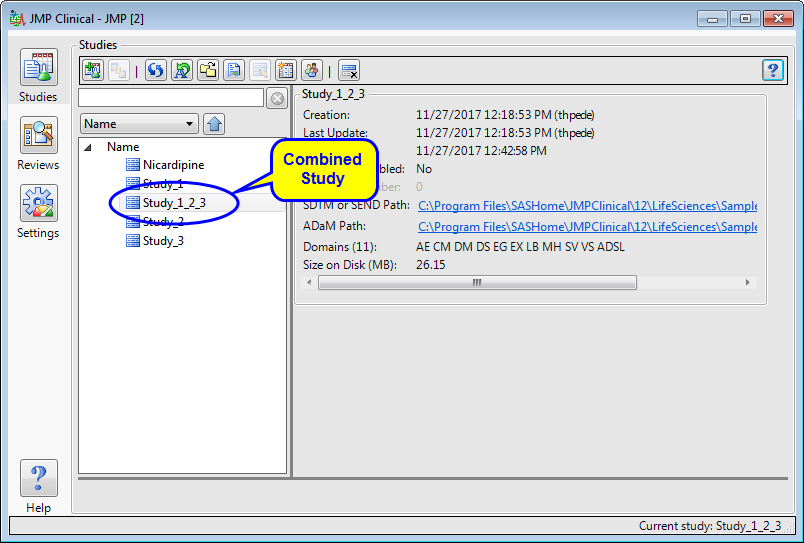
Click to complete the process and add the combined study to the main window, as shown below.
|