The AE Distribution report enables you to compare distributions of adverse events across treatment arms for subgroups defined by demographics such as age, sex and race.
Note: Refer to Distribution Reports for a description of the general analysis performed by all JMP Clinical distribution reports.
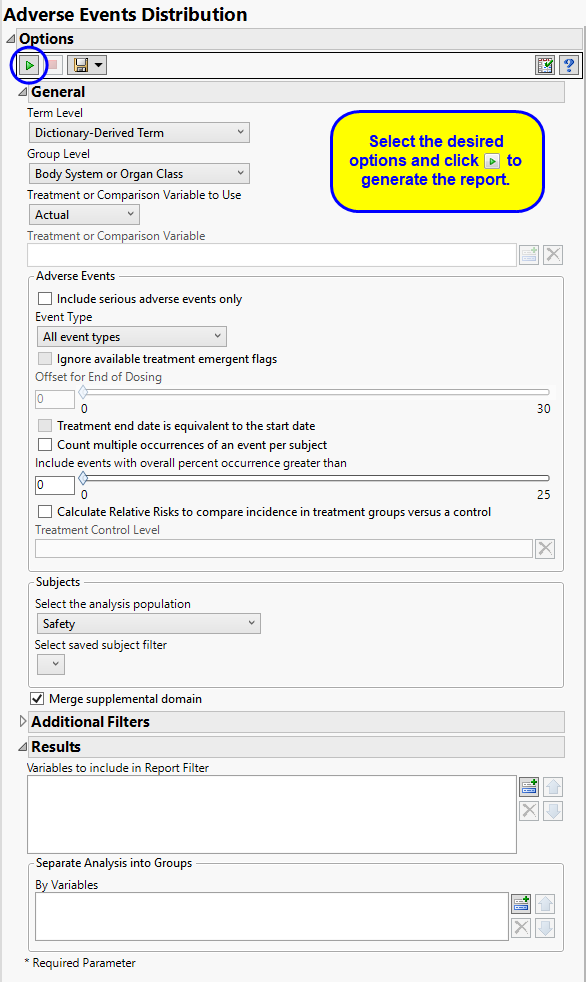
Running AE Distribution with Nicardipine using Actual for Treatment or Comparison Variable to Use generates the Report for AE Distribution as shown below. Differences with other reports are noted throughout this output description.
|
•
|
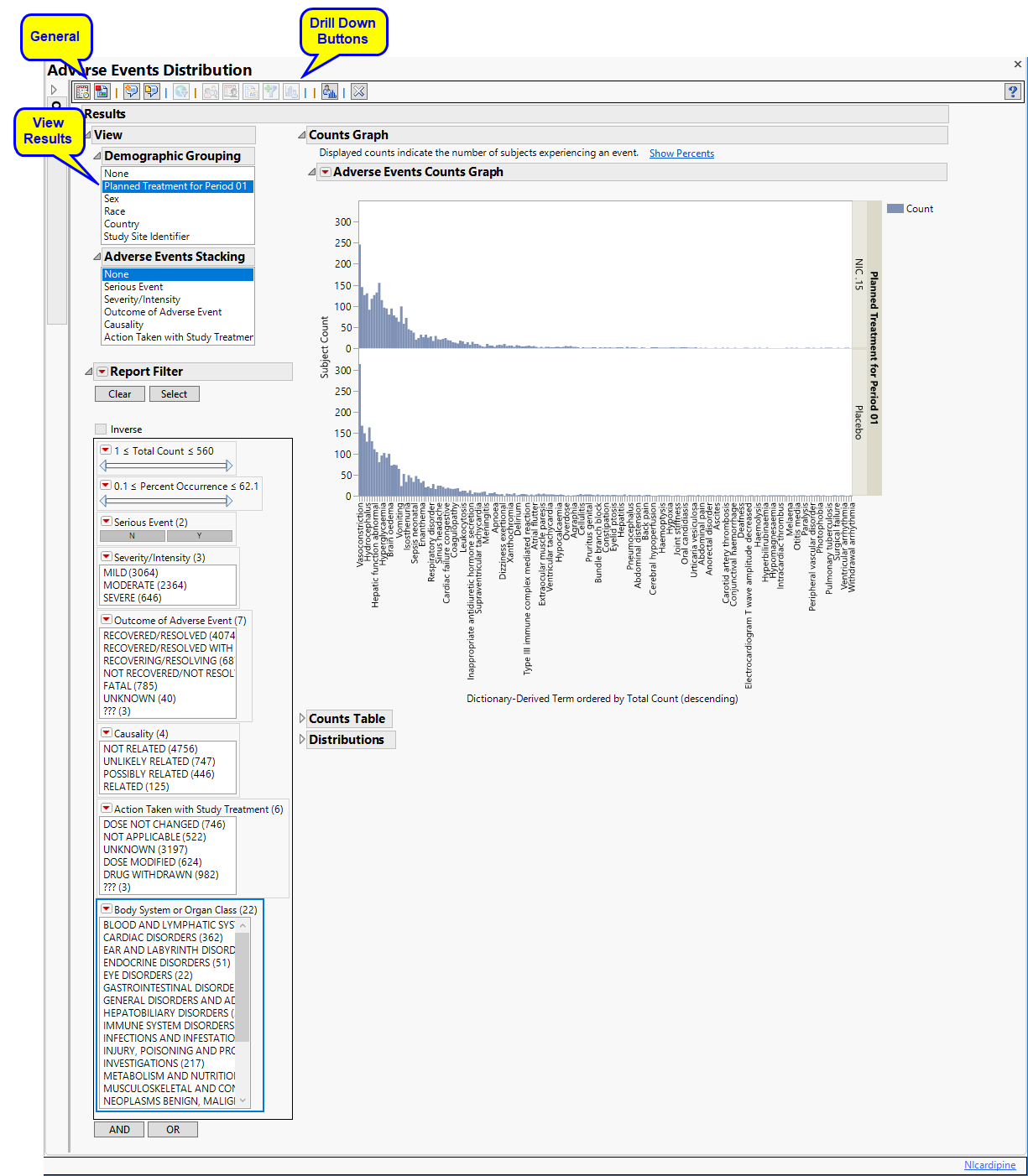
: Panel enables analysis of various demographic groups by selected AE.
|
|
•
|
: Panel enables analysis of various AEs by selected demographic group.
|
This enables you to subset subjects based on demographic characteristics and other criteria. Refer to Data Filter for more information.
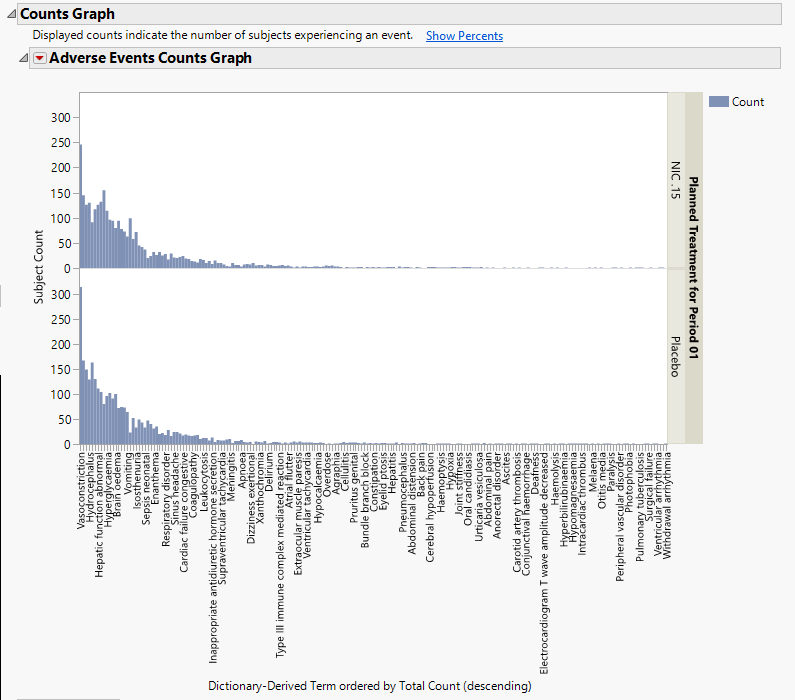
Using a Bar Chart or Tree Map, this section summarizes the distributions of terms based on xxDECOD or, if unavailable, xxTERM or xxTRT depending on CDISC domain type. Results are summarized by selected demographic grouping and can be displayed either as total counts or percentages.
If AEBODSYS is available, a Table summarizes the distribution of adverse events, body system or system organ class. Results are summarized by Trial Time Windows. For Interventions Distribution, xxCAT is used (if available) and the section name reflects the appropriate label. For example, for CM, the section is Category for Medication treemap.
The Counts Table section contains the following element:
|
•
|
One Table detailing the counts for each treatment group, for each dictionary-derived term within each body system or organ class.
|
|
•
|
One table listing total demography counts (as taken from DM during report generation NOT to be confused with the count of subjects experiencing events) that dynamically changes based on the Demographic Grouping column to display the denominators used in Percent calculations, described in Understanding Count and Percent Calculations. The values in this table are presented in terms of N, the counts of subjects from the demographic table regardless of event occurrence, and used as a reference for indicating how many subjects within a group experienced an event and as the denominators for percent calculations.
|
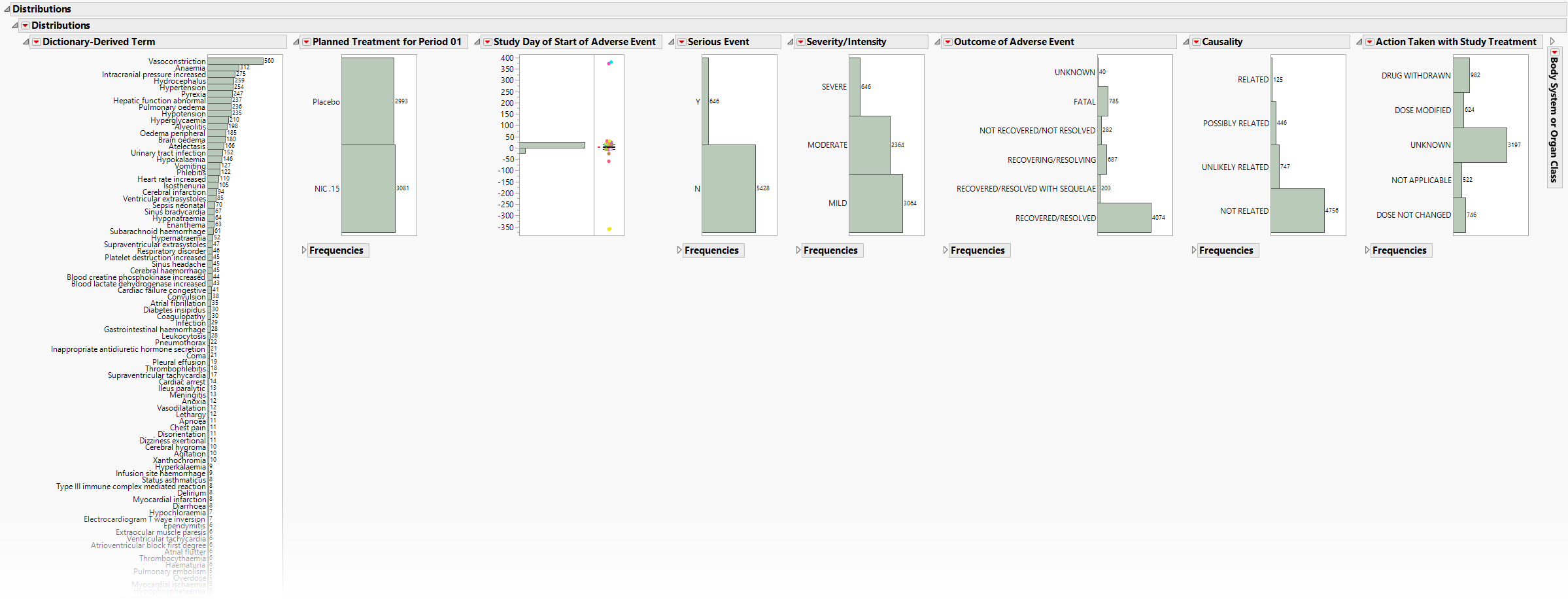
Presents Histograms of event characteristics such as Study Start Day, Causality, Outcome, Severity/Intensity, and Seriousness. Other Distribution reports present term, classification, and study day variables.
The Distributions section contains the following elements:
|
•
|
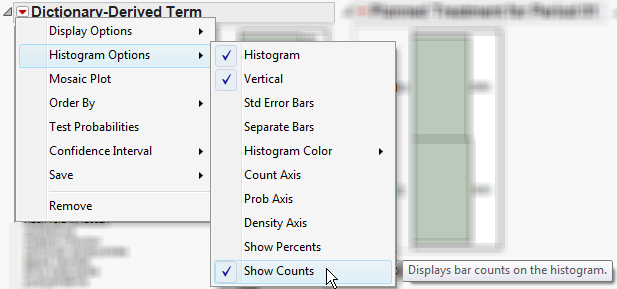
The height (or length) of each bar is an indication of the number of events exhibiting such characteristics. Numbers above (or to the right of) each bar reflect a count of the number of events. Counts or percents can be added or removed using Histogram Options, which can be viewed by selecting the red triangle ( ).
).
Covariates summarized include Study Start Day, Causality, Outcome, Severity/Intensity, and Seriousness. If Count multiple occurrences of an event per subject is not checked, the event chosen is based on sorting the data by seriousness (AESER), severity (AESEV or AETOXGR), and study day (AESTDY). For Events Distribution or Interventions Distribution, the earliest of each event or intervention is presented.
See Distributions for more information.
|
•
|
Profile Subjects: Select subjects and click
|
|
•
|
Show Subjects: Select subjects and click
|
|
•
|
Cluster Subjects: Select subjects and click
|
|
•
|
Adverse Events Narrative Generation: Select subjects and click
|
|
•
|
Demographic Counts: Select subjects and click
|
|
•
|
Demographic Counts: Select subjects and click
|
|
•
|
Related CM (AE Distribution only): Select subjects and click
|
|
•
|
Related Labs: Select subjects and click
|
|
•
|
Related Vitals: Select subjects and click
|
|
•
|
Related ECG: Select subjects and click
|
|
•
|
Related AE (Interventions Distribution only): Select subjects taking selected medications and click
|
|
•
|
Unique Occurrence Subject Counts: Select individual records and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the Options arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information.
|
Note: For information about how treatment emergent adverse events (TEAEs) are defined in JMP Clinical, please refer to How does JMP Clinical determine whether an Event Is a Treatment Emergent Adverse Event?.
Term and Group Levels are determined by the coding dictionary for the Event or Intervention domain of interest, typically these levels follow the MedDRA dictionary. You must indicate how each adverse event is named and the level at which the event is considered. For example, selecting Reported Term and Body System or Organ Class as the Term Level and Group Level, respectively, reports the event specified by the actual event term as reported in the AE domain on the affected organ or body system.
The primary goal of clinical trials is to distinguish treatment effects when reporting and analyzing trial results. Treatments are defined by specific values in the treatment or comparison variables of the CDISC models. These variables are specified in this report using the Treatment or Comparison Variable to Use andTreatment or Comparison Variable options.
Available variables include Planned, which is selected when the treatments patients received exactly match what was planned and Actual, which is selected when treatment deviates from what was planned.
You can also specify a variable other than the ARM or TRTxxP (planned treatment) or ACTARM or TRTxxA (actual treatment) from the CDISC models as a surrogate variable to serve as a comparator. Finally you can select None to plot the data without segregating it by a treatment variable.
By default, all events are included in the analysis. However, you can opt to include only those considered serious. Selecting the Include serious adverse events only option restricts the analysis to those adverse events defined as Serious under FDA guidelines.
Analysis can consider all events or only those that emerge at specific times before, during, or after the trial period. For example, selecting On treatment events as the Event Type includes only those events that occur on or after the first dose of study drug and at or before the last dose of drug (+ the offset for end of dosing).
If you choose to Ignore available treatment emergent flags, the analysis includes all adverse events that occur on or after day 1 of the study.
By default, post-treatment monitoring begins after the patient receives the last treatment. However, you might want to specify an Offset for End of Dosing, increasing the time between the end of dosing and post-treatment monitoring for treatments having an extended half-life.
Check the Treatment end date is equivalent to the start date if the treatment end date (EXTENDTC) is missing from the data. In this case, it is assumed that all treatments were given on the same day and that the treatment start date can be used instead.
By default, the number of occurrences of an adverse event is computed as the number of subjects experiencing that effect, regardless of how many times each subject experienced the effect. When a patient experiences multiple occurrences of an event, the software selects the occurrence for analysis based first on whether it is a serious event, then by severity and finally by time. When the Count multiple occurrences of an event per subject check box is checked, the counts reported for each event represent the total of all the occurrences of that event.
Use the Include events with overall percent occurrence greater than option to specify a threshold that enables you to consider only those events that exceed that threshold, in terms of overall percent of occurrence, and exclude those events that do not.
The Calculate Relative Risks to compare incidence in treatment groups versus a control and Treatment Control Level options are used in conjunction to compute the relative risk of experiencing each adverse event for those subjects receiving the experimental treatment compared to the specified control group.
Filters enable you to restrict the analysis to a specific subset of subjects and events based on values within variables. You can also filter based on population flags (Safety is selected by default) within the study data.
If there is a supplemental domain (SUPPAE) associated with your study, you can opt to merge the non-standard data contained therein into your data.
See Select the analysis population, Select saved subject Filter1, Merge supplemental domain, Include the following adverse events:, Additional Filter to include Adverse Events, Additional Filter to Include Subjects.
Use the Variables to include in Report Filter option to specify the variables to be included in the report data filter.
You can also subdivide the subjects and run analyses for distinct groups by specifying one or more By Variables.
Subject-specific filters must be created using the Create Subject Filter report prior to your analysis.