Note: JMP Clinical uses a special protocol for data including non-unique Findings test names. Refer to How does JMP Clinical handle non-unique Findings test names? for more information.
Note: Refer to Distribution Reports for a description of the general analysis performed by the JMP Clinical distribution reports.
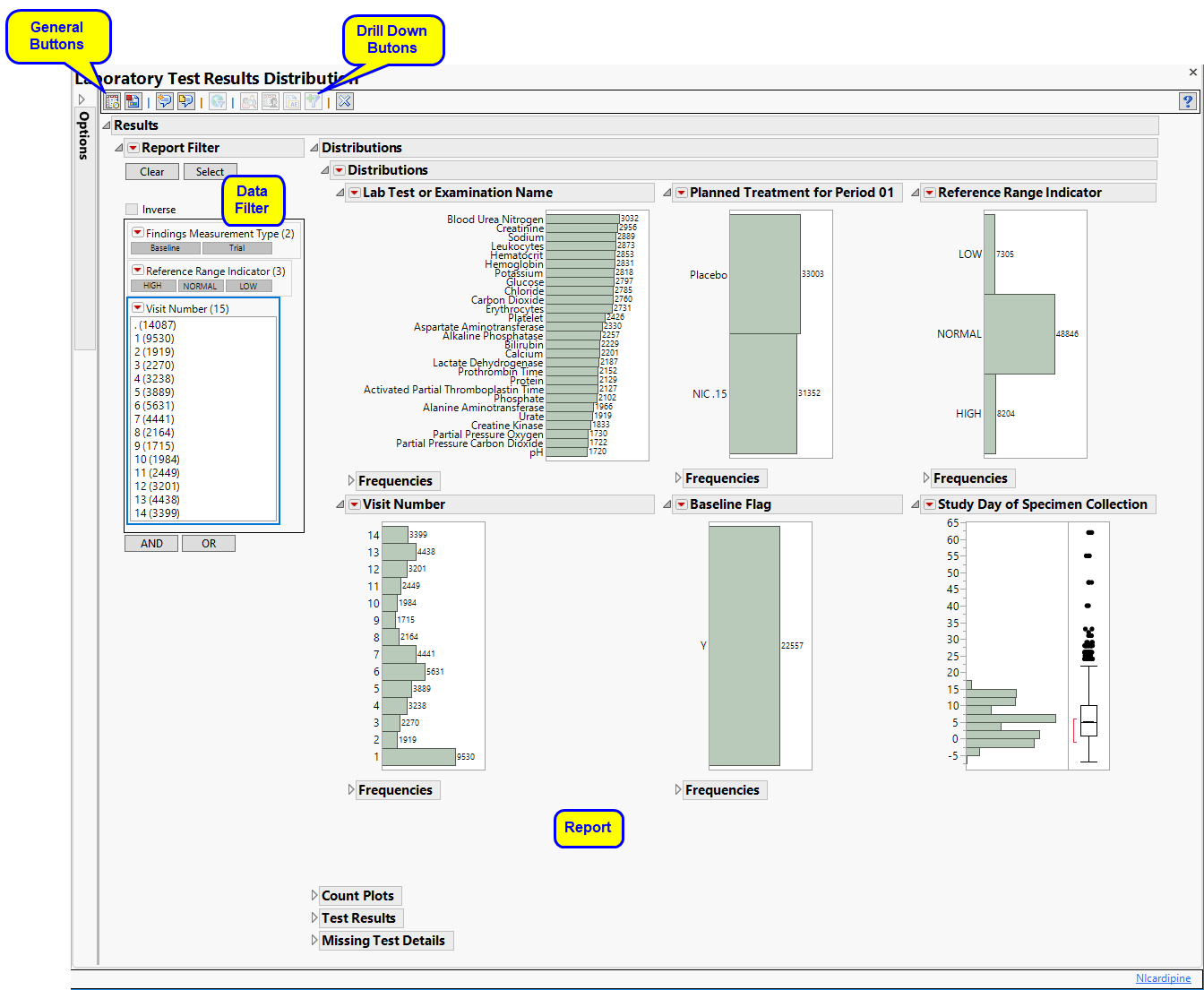
Running Findings Distribution for Nicardipine using default settings generates the Report shown below.
The Report contains the following sections:
Contains Histograms to display the distribution of Findings tests taken during the study and other relevant variables for the selected Findings domain.
|
•
|
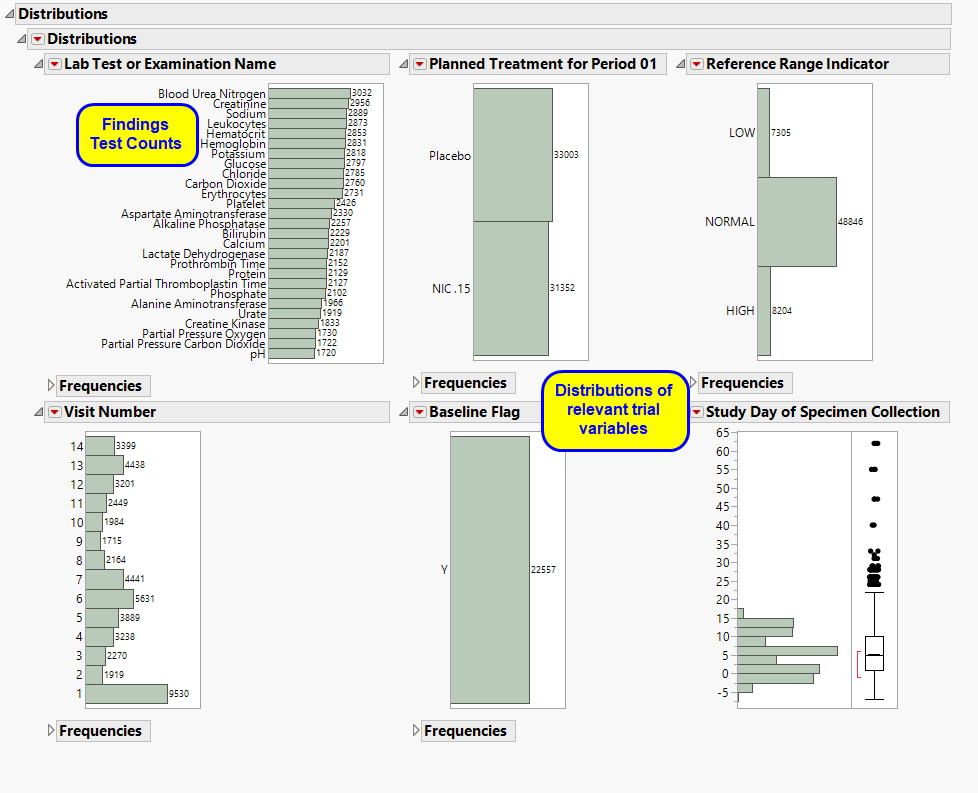
One Findings Test Counts Graph.
|
This graph shows a Histogram displaying how often measurements were taken for each findings test (xxTESTCD) during the study.
|
•
|
A set of Distributions.
|
These display counts and histograms of relevant variables in the Findings data set. A distribution of subjects on the Actual, Planned, or Specified Treatment is shown as well as other findings variables (if present). Findings variables displayed can include the Findings Body System (xxBODSYS), Reference Range Indicator (xxNRIND), the Category for the Test (xxCAT) and Subcategory (xxSCAT), the Categorical Findings Result (xxSTRESC, only displayed for categorical findings domains), the Visit and Time Points at which findings were taken (VISIT and xxTPT), Baseline Flag (xxBLFL), and the Study Day.
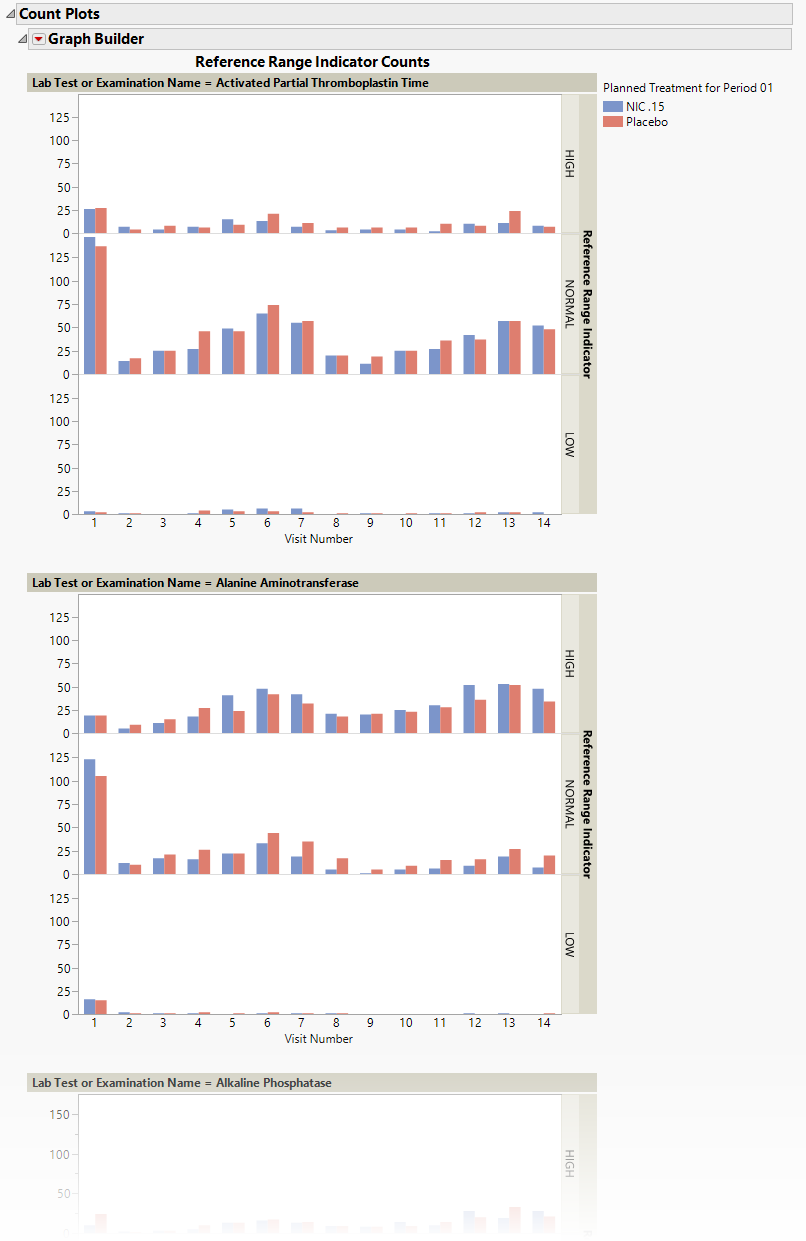
The Count Plots section is shown below. This section is shown only when the xxNRIND variable and either VISIT or VISITNUM is found in the Findings data set.
|
•
|
These plots show the distribution of measurements across categories of the Reference Range Indicator variable (xxNRIND). For example, you can see how many laboratory measurements for a given test were categorized as HIGH, NORMAL, or LOW based on values of the Reference Range Upper Limit and Reference Range Lower Limit (LBSTNRLO and LBSTNRHI, respectively). The graph contains bars representing the counts within each category for each treatment across Study Visits (VISIT or VISITNUM).
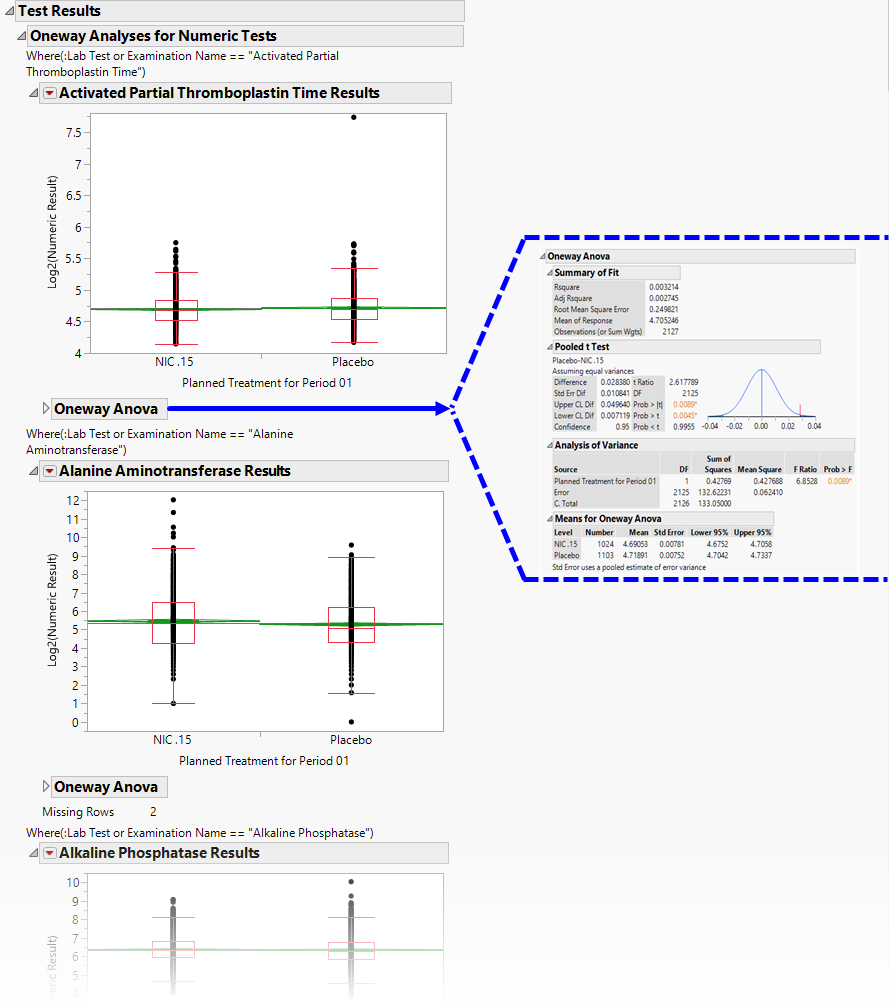
Contains One-way Analyses ( ANOVA) for each test that has numeric measurement results (xxSTRESN values), Contingency Analyses for each Findings test that has character results (xxSTRESC values but missing xxSTRESN values), or both.
Note: This section can display analyses for numeric and/or categorical findings tests, depending on the tests found within the chosen analysis Findings domain.
It contains one or both of the following elements:
|
•
|
A set of Oneway Analyses for Numeric Findings Tests.
|
Each analysis represents an Analysis of Variance ( ANOVA) for each numeric Findings test (tests that contain nonmissing values for xxSTRESN) to compare measurements taken across different treatment arms. You can click the Oneway ANOVA outline box below each plot to show the statistical results of the analysis.
Note that this analysis is across all measurements taken during the study and should be used to get an idea of possibly significant differences in measurements across treatment arms. This is a simple model. You can fit a more appropriate model that accounts for the repeated measures taken for subjects, as well as initial baseline measurements, with the Findings ANOVA report.
See the JMP Fit Y by X platform for more details about Oneway Analysis.
|
•
|
A set of Contingency Analyses for Categorical Findings Tests.
|
Each analysis represents a contingency analysis for each categorical test (tests that contain nonmissing values for xxSTRESC but are missing values for xxSTRESN) to compare measurements taken across different treatment arms. A Mosaic Plot shows the proportion of measurements for values of xxSTRESC across treatment arms. You can click the Tests outline box below each plot to display tests for significant differences in the proportion of values across treatment.
See the JMP Fit Y by X platform for more details about Contingency Analysis.
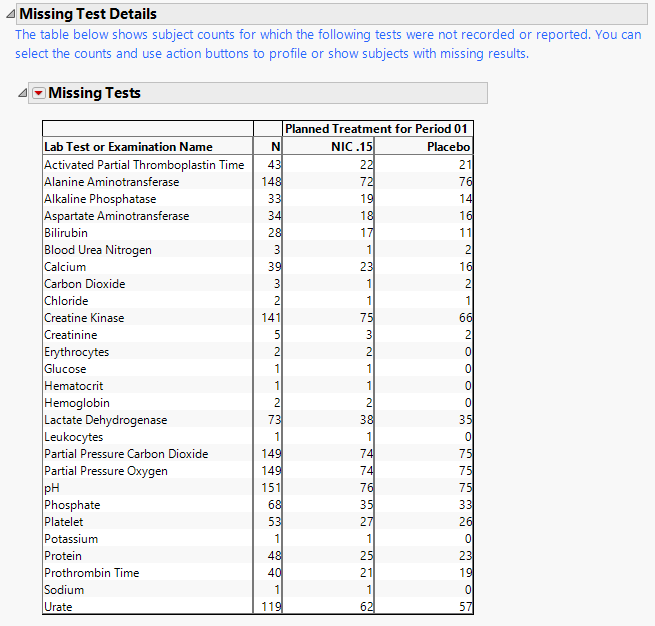
Contains tables displaying subject counts for tests that were either not recorded, or that were recorded but have missing measurement values (of xxSTRESN and/or xxSTRESC).
Note: This section is not shown if all subjects have nonmissing measurements taken for all Findings tests recorded.
|
•
|
A Missing Tests Table.
|
This table displays subject counts for which Findings tests were either not reported or not recorded. For example, the number of subjects that did not have any record taken for the ALT lab test in the LB domain is displayed in this table for the ALT test row.
This enables you to subset subjects based on demographic characteristics and study site. Refer to Data Filter for more information.
|
•
|
Profile Subjects: Select subjects and click
|
|
•
|
Show Subjects: Select subjects and click
|
|
•
|
Cluster Subjects: Select subjects and click
|
|
•
|
Demographic Counts: Select subjects and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information.
|
Use the Findings Domain to Analyze option to specify whether to plot the distribution of measurements from either the Electrocardiogram (EG), Laboratory (LB), or Vital Signs (VS) findings domains. LB is selected by default.
You can use the Findings Domain Tests for Analysis option to plot the distributions of one or more selected findings tests. Leaving the field blank (the default selection) plots the distributions for all available findings tests.
The primary goal of clinical trials is to distinguish treatment effects when reporting and analyzing trial results. Treatments are defined by specific values in the treatment or comparison variables of the CDISC models. These variables are specified in this report using the Treatment or Comparison Variable to Use andTreatment or Comparison Variable options.
Available variables include Planned, which is selected when the treatments patients received exactly match what was planned and Actual, which is selected when treatment deviates from what was planned.
You can also specify a variable other than the ARM or TRTxxP (planned treatment) or ACTARM or TRTxxA (actual treatment) from the CDISC models as a surrogate variable to serve as a comparator. Finally, you can select None to plot the data without segregating it by a treatment variable.
By default, JMP Clinical reports unaltered laboratory measurement values. In any cases, simply examining the raw numbers can make interpretation somewhat confusing. Normalization of Lab Measurements to accepted values can often ease these difficulties. JMP Clinical offers three options for normalizing your data.
Selecting LLN normalizes the data to the lower limit of the expected normal range and is best used when you expect the values to fall below the normal. Normalized values less than one are considered to be lower than normal.
Selecting ULN normalizes the data to the upper limit of the expected normal range and is best used when you expect the values to exceed the normal range. Normalized values greater than one are considered to be higher than normal.
Selecting Geometric normalizes the data such that the lower limit of the expected normal range is set to -1 and the upper limit of the expected normal range is set to +1. This method is best used when there is no expectations of where the values might fall. Normalized values less than -1 are considered to be lower than normal while values greater that +1 are higher than normal.
Log transformations can make certain response distributions closer to Gaussian with constant variance and can enable you to draw more accurate statistical conclusions under standard modeling assumptions. They are especially useful for ratio-type measurements or measurements that are always positive and skewed to the right. You can use the log Transformation of Lab Measurements options to either use non-transformed data or to log2- or log10-transform your measurements.
Note: These options are available only when LB is the specified domain.
You might or might not want to include unscheduled visits when you are analyzing findings by visit. Check the Remove unscheduled visits to exclude unscheduled visits.
Filters enable you to restrict the analysis to a specific subset of subjects and/or findings records, based on values within variables. You can also filter based on population flags (Safety is selected by default) within the study data.
If there is a supplemental domain (SUPPXX) associated with your study, you can opt to merge the non-standard data contained therein into your data.
The Subset of Visits to Analyze option enables you to restrict to a specific subset of visits.
See Select the analysis population, Select saved subject Filter1, Merge supplemental domain, Include the following findings records:, Additional Filter to Include Findings Records, Subset of Visits to Analyze and Additional Filter to Include Subjects2 or more information.
Check the Perform Oneway or Contingency Analysis option to perform a one-way analysis for continuous tests or a contingency analysis for categorical/character tests.
Use the Variables to include in Report Filter option to specify the variables to be included in the report data filter.
You can also subdivide the subjects and run analyses for distinct groups by specifying one or more By Variables.
Subject-specific filters must be created using the Create Subject Filter report prior to your analysis.
For more information about how to specify a filter using this option, see The SAS WHERE Expression.