Number of days prior to Adverse Event start date for reporting concomitant medications
Specify the number of days prior to the AE start date for reporting medications.
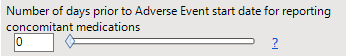
A value of 0 indicates medications that were taken the day of event onset. A value of X includes concomitant medications that were taken on the day of the onset of the AE and up to X days prior to AE onset.
To Specify the Number of Days for Reporting Concomitant Medications:
| 8 | Either enter the number of days in the text field or move the slider to the right or to the left to change the value. |
Tip: To change the scale of the slider, right-click on the slider and select from the pop-up menu. Change the upper or lower boundaries in the that appears, to rescale the slider and click .