Discontinuation Time to Event
This report creates a plot that displays the proportion of subjects who have not discontinued the study by days completed. Discontinuation events can be limited to those due to adverse events or deaths only (default) or those due to all reasons.
Report Results Description
Running this report for Nicardipine using default settings generates the tabbed Results shown below.
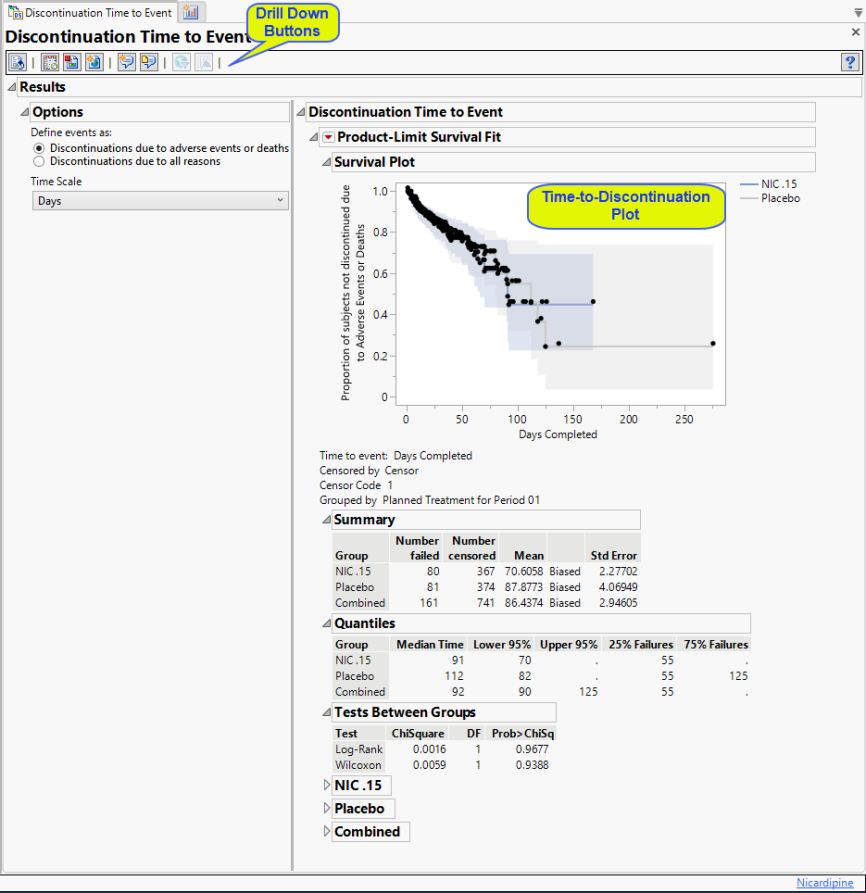
Results
Contains a Kaplan-Meier Survival Curve time-to-event analysis that compares the time until discontinuation of individuals in each treatment arm in the study. Otherwise, subjects are censored when they complete the trial. You can define what constitutes a discontinuation using the Define events as: option in the Options.
Options
Define events as:
A patient may drop out of a study, or discontinue, because of a variety of reasons. It is important to consider reasons for patient s discontinuation in a study while assessing the outcome of any study. Discontinuations directly resulting from adverse events or death arising from study protocols must absolutely be considered. Others, however, are not relevant to the outcome. For example, relocation of a patient to a different city could cause the patient to discontinue, but would not be significant and can safely be excluded from the analysis. You can use the Define events as: option to differentiate between these events.
Time Scale
By default, time is measured in days. However, you can change the Time Scale to measure time in hours, weeks, months, or years. This option is useful for assessing report graphics for exceptionally long studies.
General and Drill Down Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Click  to rerun the report using default settings. to rerun the report using default settings. |
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Subject Explorer/Review Subject Filter. to open and view the Subject Explorer/Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
Default Settings
Refer to Set Study Preferences for default Subject Level settings.
Methodology
Discontinuations are determined using the last record per subject where the disposition category is "Disposition Event." When the option for "Definte events as:" is set to Discontinuations due to adverse events or deaths, disposition terms of "DEATH", "DEAD", "DIED" and "ADVERSE EVENT" are all considered discontinuations. When the option is set to Discontinuations due to all reasons, then any disposition event not listed as "COMPLETE" or "COMPLETED" is considered a discontinuation. If a subject does not experience a discontinuation event, they are censored at the date of the last disposition event. If a disposition event does not exist for a subject, then the censor date is set to the last treatment date(TRTSDT) or the reference end date(RFENDTC). Any subjects with a missing disposition date and missing censor date are removed from the analysis and a note will appear at the top of the report to indicate subjects have been removed.