Risk Based Monitoring
This report generates a dashboard for risk-based monitoring of clinical trials.
Note: You should refer to How does JMP Clinical define various terms for risk-based monitoring? for information about how terms used in risk-based monitoring are defined.
Report Results Description
Running Risk Based Monitoring for the Nicardipine study generates the Results shown below. Output from the report is organized into sections. Each section contains one or more plots, data panels, data filters, or other elements that facilitate your analysis.
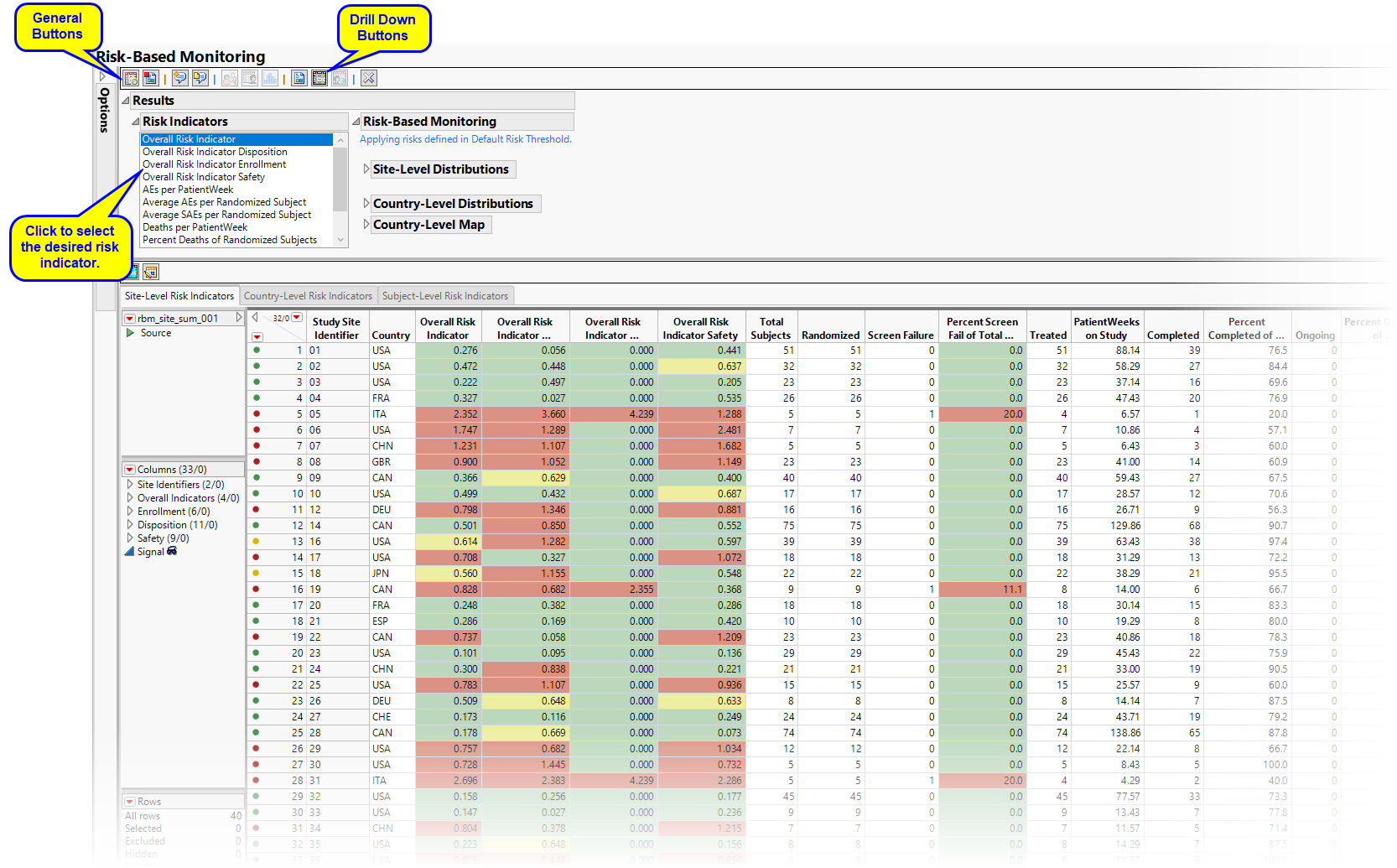
The Report contains the following elements:
Site-Level Distribution
Contains a series of Histograms for risk indicators from the site-level risk indicators data table.
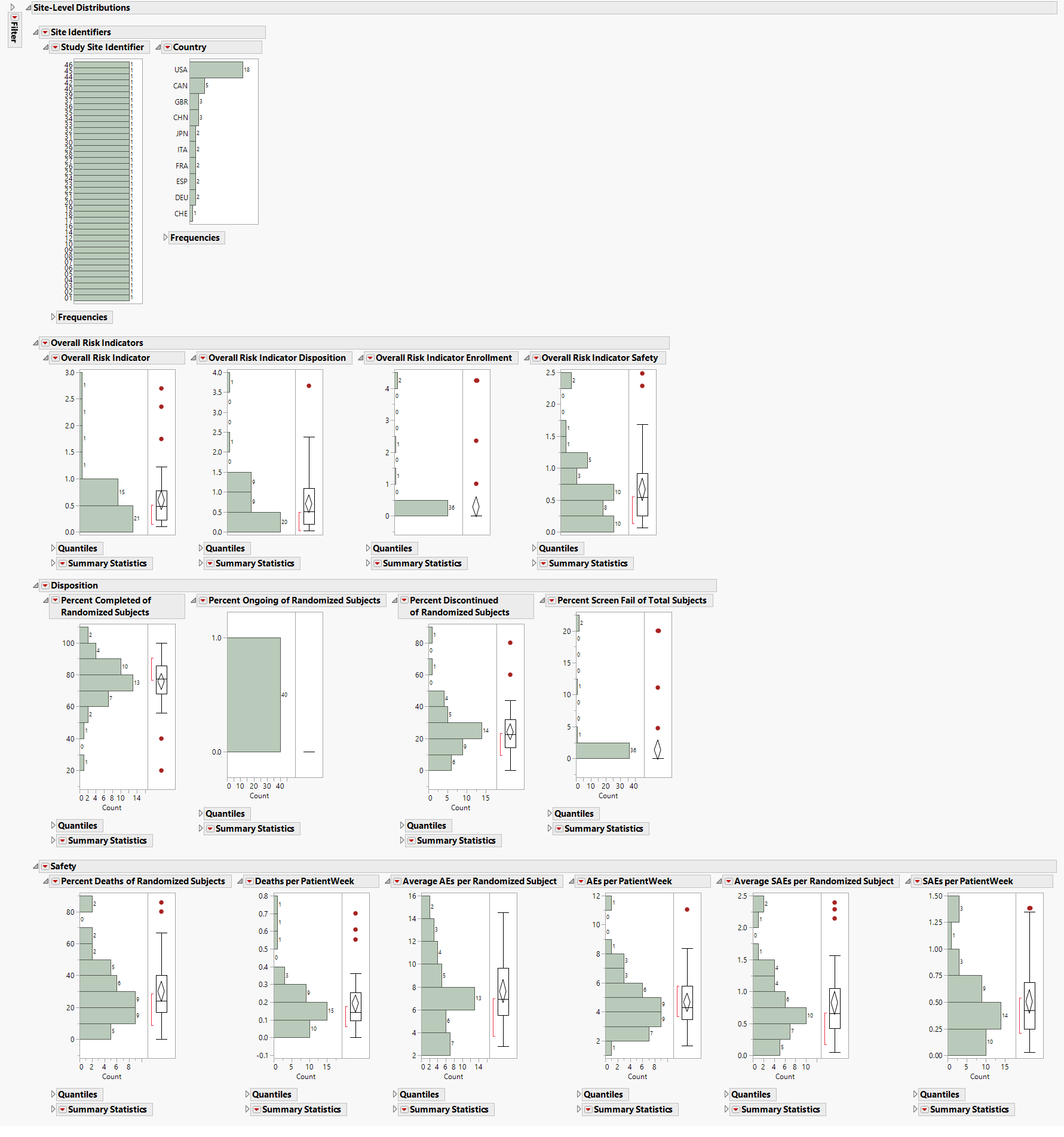
The Site-Level Distributions section contains the following elements:
| • | A set of Histograms for variables grouped by Site Identifiers, Overall Indicators, Enrollment Metrics, Disposition, Adverse Events, and Manually-Entered Site Metrics. This section enables you to view the distribution of risk indicators across sites, possibly identifying any outliers. |
The color of outliers is based on the risk indicator selected from the Risk Indicators down.
See Distributions in the JMP documentation for more information.
| • | A Local Data Filter to subset histograms to data of interest, for example, to data for a particular set of study monitors. |
Site-Level Maps
Contains global and, depending on the countries present in the data, country maps detailing the location of the clinical sites based on geocoding. Site colors are based on the selected risk indicator. Note; This section is present only with geocoded sites.
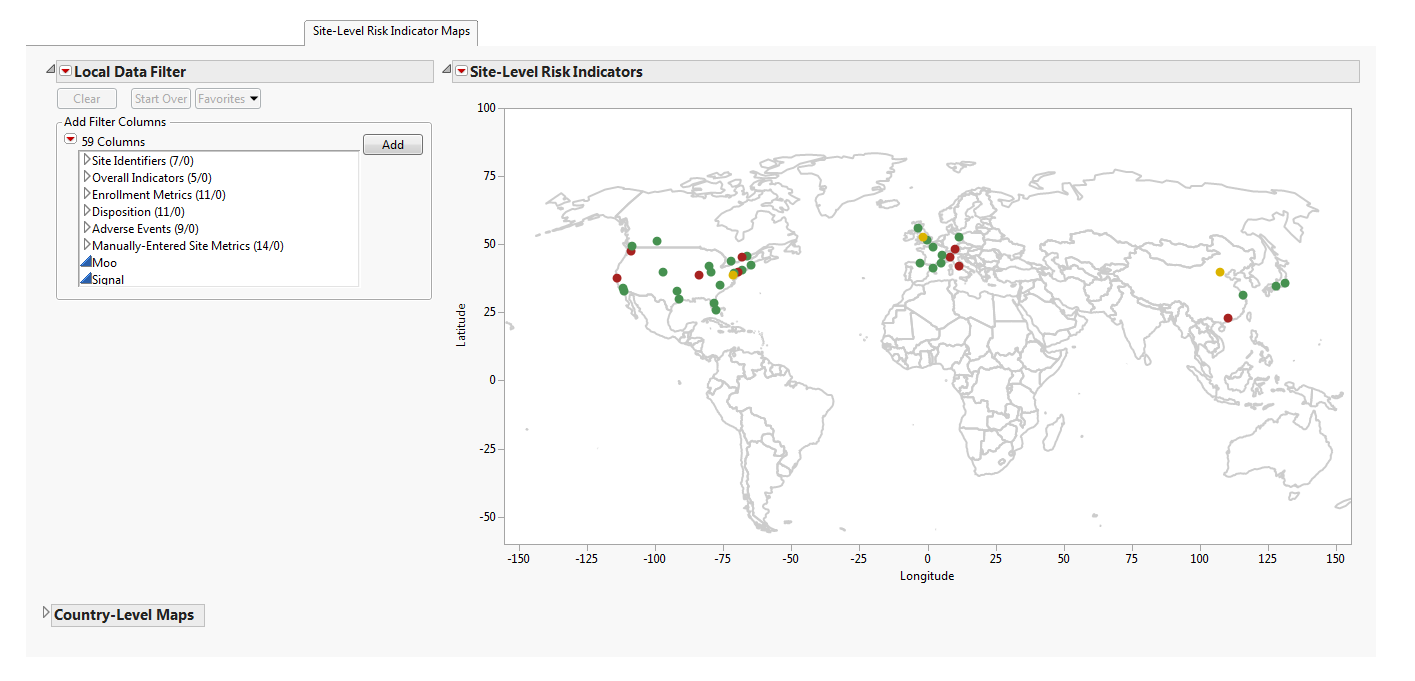
The Site-Level Maps section contains the following elements:
| • | Maps: A global map is provided detailing the location of clinical sites based on the available geocoding. The colors of the site markers are based on the risk indicator selected from the Risk Indicators down. These colors enable you to identify if a risk is attributable to specific locations. Maps at the country level are available for the United States, Canada, France, Germany, Great Britain, Italy, Spain, China, and Japan. Note: Maps are provided only for these countries if they are present in the study database. |
See Maps in the JMP documentation for more information.
| • | A Local Data Filter to subset histograms to data of interest, for example, to data for a particular set of study monitors. |
See Local Data Filter in the JMP documentation for more information.
Country-Level Distributions
Contains a series of Histograms for risk indicators from the country-level risk indicators data table.
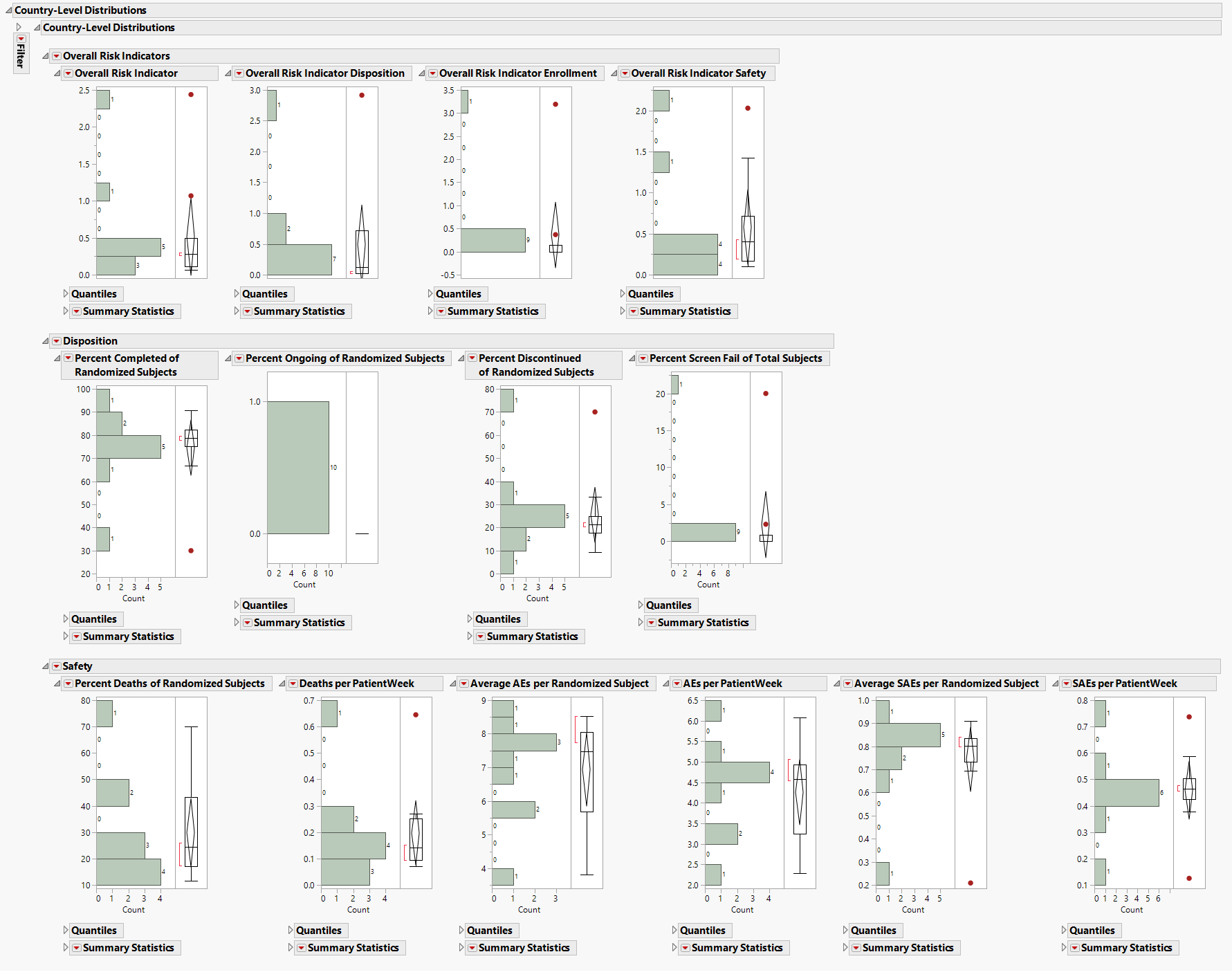
| • | A set of Histograms for variables grouped by Overall Indicators, Enrollment Metrics, Disposition, Adverse Events, and Manually-Entered Site Metrics. |
This section enables you to view the distribution of risk indicators across countries, possibly identifying any outliers. The color of outliers is based on the risk indicator selected from the Risk Indicators down.
See Distributions in the JMP documentation for more information.
| • | A Local Data Filter to subset histograms to data of interest (for example, to data for a particular region). |
See Local Data Filter in the JMP documentation for more information.
Country-Level Map
Contains global map coloring countries based on the selected risk indicator.
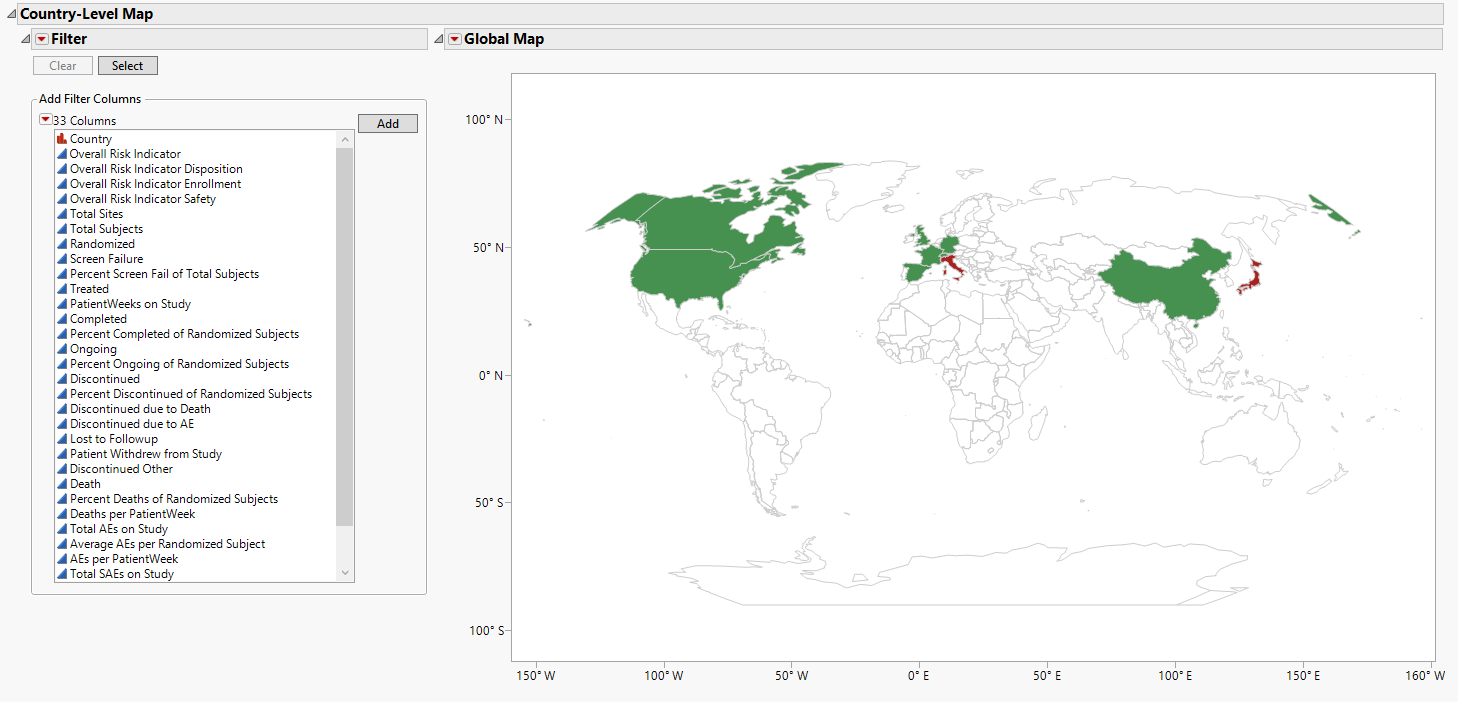
The Country-Level Map section contains the following elements:
| • | Maps: A global map is provided detailing risk indicators at the country level. The color of country is based on the risk indicator selected from the Risk Indicators down. These colors enable you to identify if a risk is attributable to specific locations. |
See Maps in the JMP documentation for more information.
| • | A Local Data Filter to subset histograms to data of interest (for example, to data for a particular set of study monitors). |
See Local Data Filter in the JMP documentation for more information.
Risk Indicators
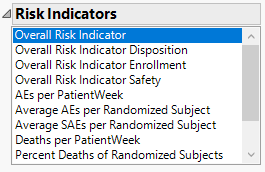
Click to highlight risk-indicator colors on data table row markers, histogram outliers, and map points. Note: This option is for use with indicators having defined thresholds,
Note: Cells with missing values do not display
Output Data
| • | Site-Level Data: This data table displays risk indicators derived using the study database as well data included in the supplemental data set. Updated disk indicators can be supplies either by using the Update Study Risk Data Set option or supplemental data sets (See How Supplemental Data Sets are Used by JMP Clinical. for more information.). Risk indicators with defined thresholds are colored in green, yellow, or red. Note: The Signal column is the Risk Score for that particular Risk Indicator. |
| • | Country-Level Data: This data table displays risk indicators summarized at the country level. Risk indicators with defined thresholds are colored in green, yellow, or red. Note: The Signal column is the Risk Score for that particular Risk Indicator. |
| • | Subject Summary Data: This data table summarizes the status of the study subjects. |
Action Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Profile Subjects: Select subjects and click  to generate the patient profiles. See Profile Subjects for additional information. to generate the patient profiles. See Profile Subjects for additional information. |
| • | Show Subjects: Select subjects and click  to open a data table of subjects from the sites selected from the site-level data table for review. to open a data table of subjects from the sites selected from the site-level data table for review. |
| • | Report Actions: Click  to open a set of distributions for selected rows listing recommended actions for selected study sites. to open a set of distributions for selected rows listing recommended actions for selected study sites. |
| • | Open Edit Checks: Click  to open a document of consistency checks performed on the risk indicators. The findings might suggest queries to one or more sites. to open a document of consistency checks performed on the risk indicators. The findings might suggest queries to one or more sites. |
| • | Select Rows Using Risk Indicators: Click  to select rows based on the color of the selected Risk Indicator for both the site- and country-level risk indicator data tables. to select rows based on the color of the selected Risk Indicator for both the site- and country-level risk indicator data tables. |
| • | Select Sites Using Subgroup Selection: Click  to select rows in the site-level data table for sites within the countries selected in the country-level data table. to select rows in the site-level data table for sites within the countries selected in the country-level data table. |
General
| • | Click  to view the associated data tables. Refer to View Data for more information. to view the associated data tables. Refer to View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click the arrow to reopen the completed report dialog used to generate this output. |
| • | Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information. |
Methodology
No statistical testing is performed. Moderate and severe risk are defined as described below:
When percentages are specified, risk is defined as:
| • | moderate (yellow), when the |
| • | severe (red), when |
where is the mean, median or user-supplied center value, and xij is the value of the ith site (or country, vendor, monitor) for the jth risk indicator. The quantity cij = |xij - mij|, (xij - mij, or -(xij - mij) for Direction equal to B, U, or L, respectively.
When percentages are not specified, risk is defined as:
| • | moderate (yellow), when |
| • | severe (red), when |
Overall risk indicators are calculated as 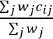 , where
, where ,
, or
for Direction equal to B, U, or L, respectively.The wj are weights for the overall risk indicator; 1 by default.
Refer to How are risk thresholds defined? for more information.
Report Options
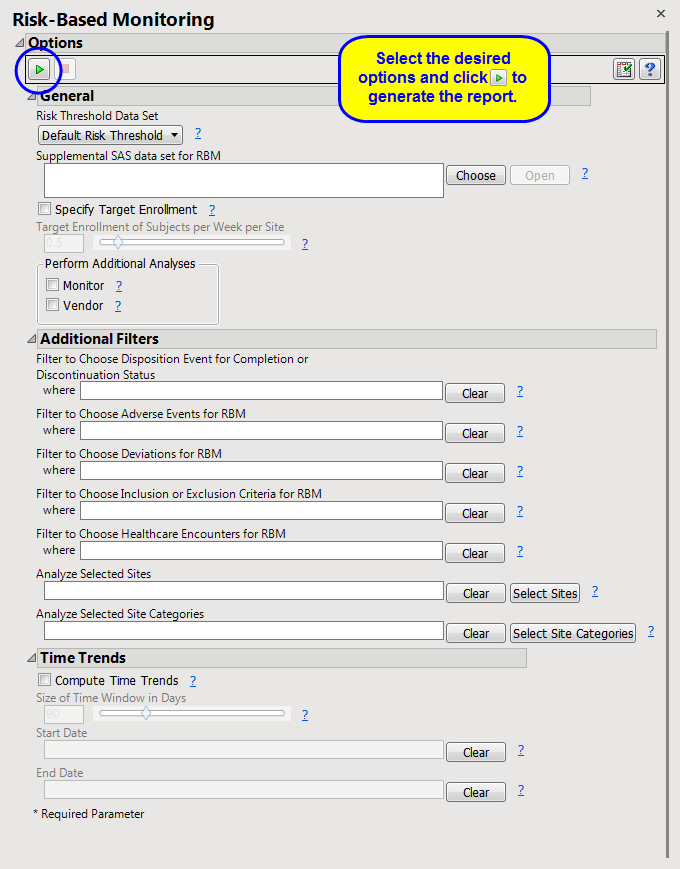
General
Risk Threshold Data Set, Supplemental SAS data set for RBM
Specify Target Enrollment, Target Enrollment of Subjects per Week per Site
Perform Additional Analyses:Monitor, Vendor
Additional Filters
Filter to Choose Disposition Event for Completion or Discontinuation Status, Filter to Choose Adverse Events for RBM, Filter to Choose Deviations for RBM, Filter to Choose Inclusion or Exclusion Criteria for RBM, Filter to Choose Healthcare Encounters for RBM
Analyze Selected Sites, Analyze Selected Site Categories
Time Trends
Compute Time Trends, Size of Time Window in Days, Start Date, End Date