Progression Free Survival
Progression free survival is used to describe how long patients can go without the disease without getting worse. This process uses a modified survival plot and hazard ratio plot to compare the number and percentage of patients who have tumor progression or death, with patients who show no tumor progression or death at the cut-off date. Results are summarized in a set of tables.
Report Results Description
Running this report for a modified Nicardipine study using default settings generates the report shown below.
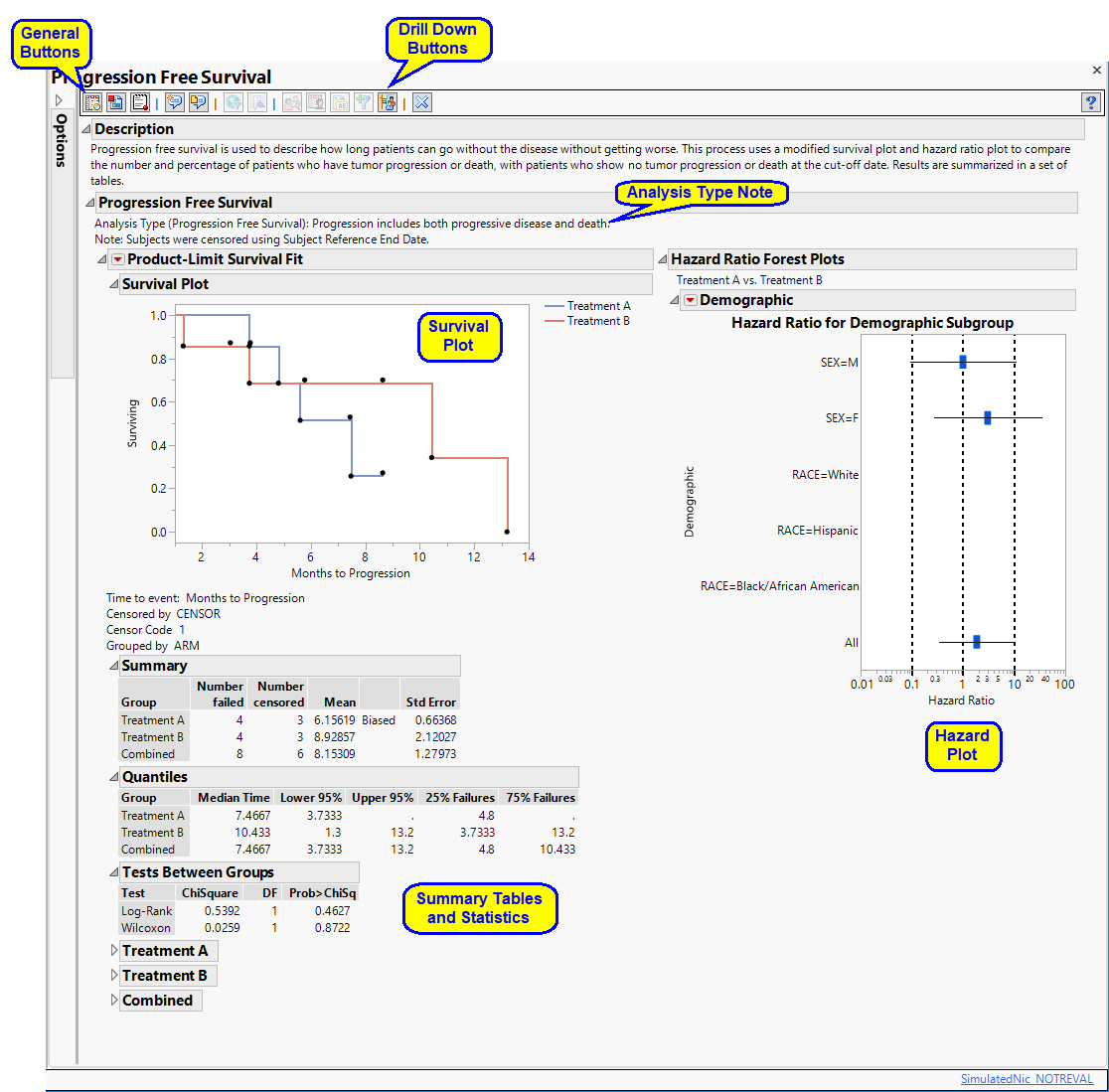
It contains the following elements:
| • | Summary text |
Summarizes the subjects plotted below.
| • | One Survival Plot |
This plot shows the probability of patients showing no disease progression over the course of the study. The plateaus indicate that the probability of not progressing remains the same during that period. The dots indicate that patients have either shown disease progression or have left the study (withdrawal or death). The connecting lines are colored by treatment.
| • | One Hazard Ratio Event Plot |
This plot compares hazard ratio confidence intervals between two different treatment regimes on subjects remaining progression free. It is based upon the Cox proportional hazards model. (See The SAS PHREG Procedure for more information.)
Each bar represents the hazard rate posed by one treatment divided by the hazard rate caused by the second treatment. In plot shown above, patients receiving the treatment (Treatment 1) or a placebo (Treatment 2) are plotted by demographic group.
Each blue rectangle represents the overall ratio for all patients in the group. Horizontal bars represent the 95% confidence interval. The ratios are plotted on a log-scale; a ratio of 1 is equivalent to no difference in hazard rates between the two treatments. Ratios greater than one show increased hazard rates resulting from treatment 1, whereas ratios less than one show increased hazard rates resulting from treatment 2.
| • | Associated Summary tables |
The tables summarize the response and statistics of patients receiving the treatment or control.
Action Buttons
| • | Profile Subjects: Select subjects and click  to generate the patient profiles. See Profile Subjects for additional information. to generate the patient profiles. See Profile Subjects for additional information. |
| • | Show Subjects: Select subjects and click  to open the ADSL (or DM if ADSL is unavailable) of selected subjects. to open the ADSL (or DM if ADSL is unavailable) of selected subjects. |
| • | Adverse Events Narrative Generation: Select subjects and click  to open the Adverse Events Narrative dialog. From this dialog, you can customize options and generate a narrative. to open the Adverse Events Narrative dialog. From this dialog, you can customize options and generate a narrative. |
| • | Create Subject Filter: Click  to create a new local subject filter. to create a new local subject filter. |
| • | Show Patients at Risk:Click  to display the number of patients at risk for progression over the course of the treatment. to display the number of patients at risk for progression over the course of the treatment. |
General
| • | Click  to view the associated data tables. Refer to View Data for more information. to view the associated data tables. Refer to View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Subject Explorer/Review Subject Filter. to open and view the Subject Explorer/Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
| • | Click the arrow to reopen the completed report dialog used to generate this output. |
| • | Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information. |
Report Options
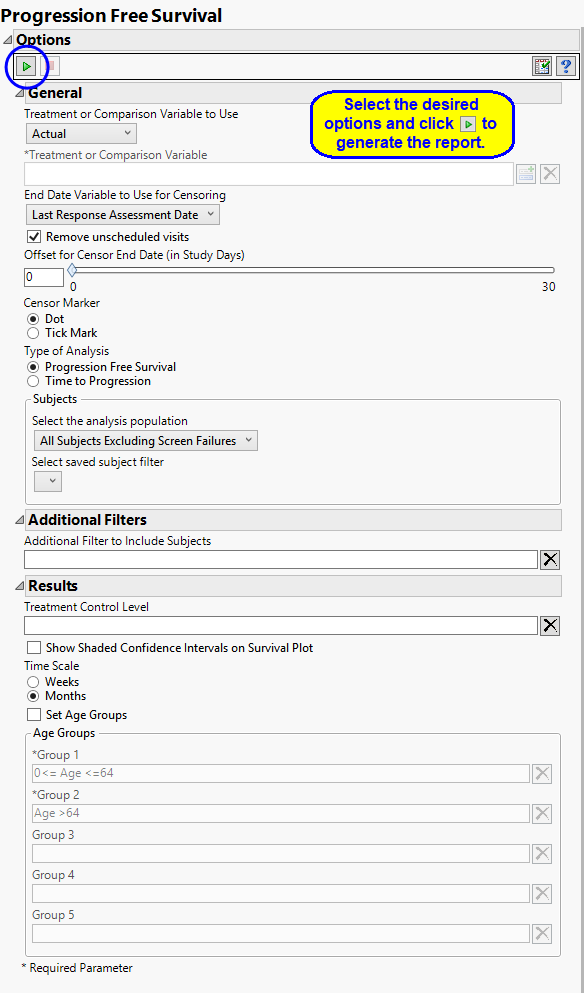
General
The primary goal of clinical trials is to distinguish treatment effects when reporting and analyzing trial results. Treatments are defined by specific values in the treatment or comparison variables of the CDISC models. These variables are specified in this report using the Treatment or Comparison Variable to Use and Treatment or Comparison Variable options.
Distributions of the specified treatment or comparison variables are shown in the output.
Available variables include Planned, which is selected when the treatments patients received exactly match what was planned and Actual, which is selected when treatment deviates from what was planned.
You can also specify a variable other than the ARM or TRTxxP (planned treatment) or ACTARM or TRTxxA (actual treatment) from the CDISC models as a surrogate variable to serve as a comparator.
See Treatment or Comparison Variable to Use, Treatment or Comparison Variable for more information.
For patients who survive progression-free beyond the end of the study, the ultimate progression-free survival values for these individuals are unknown. All that can be said is that progression-free survival exceeds the period of the study; these data are considered censored. For these cases, you must specify the end date for collecting data to assess progression. The End Date Variable to Use for Censoring option is used to select which variable date reference from the demography domain to use for calculating the censored disease progression values. Patients who progress after this date are not considered as progressing. The Last Response Assessment Date is chosen by default.
Unscheduled visits can occur for a variety of reasons. By default, these are excluded from this analysis. However, by unchecking the Remove unscheduled visits box, you have the option of including them.
The Offset for Censor End Date (in Study Days) option enables you to specify a minimum time interval following censor end date that can be used to determine whether progression has occurred. Patient who progress within that threshold are considered to have progressed whereas patients who progress after the specified threshold are not.
The Censor Marker option enables you to specify how to indicate censoring on the output plots.
Type of Analysis
The Type of Analysis option enables you to specify whether you want to measure Progression Free Survival or Time to Progression. Progression free survival measures the time from patient randomization to either tumor progression or death. Time to progression censors death and only considers objective tumor progression. A note is added to the top of the report indication which type of analysis has been selected.
Filtering the Data:
Filters enable you to restrict the analysis to a specific subset of subjects, based on values within variables. You can also filter based on population flags within the study data.
SeeSelect the analysis population, Select saved subject filter, and Additional Filter to Include Subjects, for more information.
Results
The Treatment Control Level is specified as either “Placebo” or “Pbo”, depending on the value found in your data, by default. However, if your control is defined differently you can use the text box to specify how the control level is identified in your study.
Selecting the Show Shaded Confidence Intervals on Survival Plot option adds confidence intervals to the plots.
By default, time is measured in months. However, you can change the Time Scale to plot time in either months or weeks. This option is useful for assessing report graphics for exceptionally long studies.
Factors other than treatment can influence mortality of study subjects. For example, elderly subjects might be more likely to die during or soon after participating in the study. By default, this report does not consider subject age in the analysis. However, this report enables you to Set Age Groups in order to assess subject age on mortality rates in up to 5 age groups observed in your study. See Group 1, Group 2, Group 3, Group 4, Group 5 for more information.